New Crown Pneumonia Monitoring Plan
Author:Henan Province Health and Heal Time:2022.07.18

New Crown Pneumonia Monitoring Plan
In order to guide the monitoring of new crown pneumonia in various places, implement early discovery and early reports to effectively prevent the spread of epidemic caused by overseas input cases and the rebound of domestic epidemic, consolidate the current prevention and control results, and formulate this plan.
1. Monitoring goals
(1) Timely discover and report new coronal virus infection and clustering epidemic, and take prevention and control measures early to prevent the epidemic from spreading.
(2) Dynamic monitoring virus variation, understand the effects of virus mutation on nucleic acid test agents and vaccine protection effects.
2. Monitoring and definition
(1) Positive staff of nucleic acid.
New crown virus nucleic acid testing first positive.
(2) Confirmation cases and suspected cases.
The definition of confirmed cases and suspected cases refer to the new type of coronary virus pneumonia.
(3) Announced asymptomatic infection.
The original test of the new crown virus disease is positive and has no related clinical manifestations, such as fever, dry cough, fatigue, sore throat, smell (taste) consciousness, diarrhea, etc. self -perception or clinical identification symptoms and signs, and CT imaging has no new crown crown Pneumonia imaging characteristics.
Third, people, things and environmental monitoring
(1) Monitoring of medical institutions.
Medical institutions at all levels, especially grass -roots medical and health institutions, should raise awareness of the discovery and reporting of new crown pneumonia cases, especially the following situations.
1. Strengthen the monitoring of symptoms such as fever, dry cough, fatigue, sore throat, smell, smell (taste), diarrhea and other symptoms, and conduct new crown virus nucleic acid testing for all fever patients. Those who have symptoms such as dry cough, fatigue, fatigue, sore throat, smell (taste), diarrhea, and diarrhea have a history of new crown pneumonia, or suspicious patients who are engaged in risk -risk professionals (see the classification of risk career) Timely nucleic acid detection.
2. Carry out nucleic acid testing for unknown causes of severe acute respiratory infections in unknown causes of pneumonia and hospitalization.
3. Carry out nucleic acid testing for all new patients and their accompanying personnel.
After the community health service stations, village clinics and individual clinics are found suspicious patients, they must report the community health service center or township health centers within 2 hours, and implement the "village report, township sampling, county testing" nucleic acid testing strategy, and can carry out the antigen simultaneously simultaneously Detective and found outbreak as soon as possible.
(2) Monitoring of risk career.
For personnel who are in direct contact with entry personnel, items, and environment (such as cross -border transportation tools, cleaning, maintenance, etc., port imported items, customs, and immigration management departments directly contact the inbound personnel and first -tier personnel, etc.) The staff of the centralized isolation venue, designated medical institutions and ordinary medical institutions issued hot nurtaric acid testing once a day.
Practitioners who are densely intensive, frequent contact personnel, and strong liquidity (such as express delivery, takeaway, hotel services, decoration and loading services, transportation services, shopping mall supermarkets and agricultural (collection) market staff, etc.), shopping mall supermarkets, etc.) Personnel and ordinary medical institutions have conducted two nucleic acid tests twice a week except the popular nursing clinics. After a local epidemic in the area or above appears, nucleic acid testing is performed according to the risk of the epidemic diffusion or the local epidemic prevention and control requirements.
(3) Monitoring of key institutions and personnel.
Schools and child care institutions, children's welfare field service agencies, psychiatric hospitals, training institutions and other key institutions, regulatory places, production workshops, construction sites and other dense places such as regulatory places, production workshops, construction sites, etc. The symptoms of relevant personnel should be performed under normalization. After a local epidemic appears in the jurisdiction, it shall be organized once in a timely manner
The test of the whole member nucleic acid can be carried out according to the test results and the risk of the epidemic diffusion according to the daily sampling ratio or the test requirements of the area of the area.
See Table 1 for details of risk professional personnel and personnel at key institutions.
(4) Monitoring of community management crowd.
Those who are included in the community management of the new crown pneumonians and their co -residences will conduct a nucleic acid test at the 3rd and 7th day after the discharge (cabin); In accordance with prevention and control requirements, nucleic acid detection and health monitoring.
(5) Monitoring of items and environmental monitoring.
1. Imported items and environment. Carry out sampling nucleic acid testing for imported cold chain foods and their processing, transportation, storage, sales and other places; imported goods and their cargo compartment, container, carriage, container and cargo storage venues from high -risk countries and low -temperature transportation environments are carried out. The sampling nucleic acid detection can increase the detection frequency and the number of sampling at low temperature conditions in winter. Focus on the inner and outer packaging surface of imported cold chain foods or imported goods, as well as more contact frequencies such as transportation tools, refrigerators, cold storage, warehouses, cargo compartment, container, carriage, container, etc. Sewage monitoring is carried out regularly for large -scale imported frozen items processing places.
2. Medical institutions. The nucleic acid testing of medical institutions with popular nursing clinics is regularly carried out. Focus on sampling testing for door handles, diagnosis tables, and inspection equipment such as high -risk environments such as popular risk environments.
3. Farmers (collective) markets. Nucleic acid testing is regularly carried out on the environment of large farmers (collectives) markets with cold chain food wholesale sales. Focus on sampling and detection of cold chain food stalls, storage places and sewage.
(6) Monitoring of centralized isolation venues.
During the opening period of concentrated isolation venues, environmental nucleic acid testing is carried out regularly. Focus on sampling testing of living areas, staff channels and quarantine channel handles, garbage, countertops, cleaning tools and other parts. Before the centralized isolation personnel should be collected, the items environment in the isolated room (including the surface of the mobile phone, the luggage items, the pillow surface, the bathroom door handle, etc.) should be collected for nucleic acid testing. (7) Drug monitoring
After a local epidemic, pharmacies in the jurisdiction responded to those who purchased antiviral, antiviral, antibiotics, cough and colds and other drugs for real -name registration and pushed the management of streets (communities) in the area in the area. Test.
Fourth, pathogen monitoring
(1) Sequence of virus whole genome.
Specifications for all overseas input cases, input items and related environmental positive specimens of all overseas input cases with nucleic acid detection CT value, first or early cases in local epidemic, key cases associated with epidemiological associations with early cases, local cases with unknown sources of infection sources The specimen and vaccine are sequenced after the vaccine is vaccinated.
(2) Separation of virus.
Specifications for all overseas input cases with CT value ≤30, the first or early cases in the local epidemic, key cases associated with epidemiological associations with early cases, local cases with unknown sources of infection, and nucleic acid -positive specimens after vaccine vaccination Carry out virus separation training.
(3) Management requirements.
1. Positive specimen preservation requirements. All provincial-level illness and control institutions shall uniformly deploy the preservation of all new crown virus nucleic acid-positive specimens in this province. Nucleic acid testing positive specimens shall be kept for a long time at -70 ° C/counter. The negative specimens shall be properly handled by each unit after verification.
2. Specification review. Provincial disease control institutions shall promptly report the results of the newly discovered variable -based genome sequences and the specimen to the China Centers for Disease Control and Prevention for analysis and review.
3. Regularly report. The China Centers for Disease Control and Prevention is responsible for summarizing and notifying the monitoring work of various provinces.
Fifth, mutant plants affect monitoring
(1) Monitoring object.
All new coronal virus mutations in my country, as well as the "Variant of Interest (VOI) and" VOC "(VOI) determined by all World Health Organization (WHO). The characteristics of these two types of mutation strains are:
1. Pay attention to mutation strains: refers to community transmission or clustered epidemic, or virus mutant strains found in many countries. Compared with the early reference strains, the phenotype of the mutant strain virus changes, or the amino acid mutation may cause changes in the virus phenotype.
2. Relaxing mutant strains: refers to the new crown virus that can be found in the monitoring process that may cause the virus propagation, increase the poisoning power, increase the clinical condition of the patient, or reduce the effectiveness of the existing diagnosis, treatment of drugs and vaccines, etc. Mutation strains.
(2) Monitoring requirements.
1. Provincial disease control institutions with assessment capabilities should conduct the assessment of the impact of the new coronary virus nucleic acid test agents and vaccine protection effects on the newly discovered new coronary virus variable strains found in this province. In the provinces that do not have evaluation capabilities, the specimen can be sent to the Chinese Disease Control Center virus disease in the China Disease Control Center to conduct an assessment of the effects of nucleic acid test agents and vaccine protection effects.
2. When the China Centers for Disease Control and Prevention finds the sensitivity of the detection reagent and the protection effect of the vaccine, the relevant situation shall be reported to the comprehensive group of the State Council's joint prevention and control mechanism in time.
3. For mutant strains that affect the sensitivity of the detection reagent, the China Disease Control Center virus disease and the provincial -level disease control institution laboratory with conditions shall promptly establish a specific nucleic acid detection method based on the variable plant nucleic acid sequence.
6. Monitoring information report
(1) Case information reports and ordering.
All kinds of medical and health institutions at all levels have found that nucleic acid preliminary sieve -positive personnel should conduct a preliminary sieve positive report within 2 hours after the test results are issued. Obstacles to designated medical institutions or square cabin hospitals. All report cases shall fill in "case classification" and select "suspected cases" or "confirmed cases". After receiving the report, the medical and control institutions should investigate and verify immediately, and complete the third -level confirmation review of the report information through the network direct reporting system within 2 hours. Medical institutions that do not have direct online reporting conditions shall immediately report to the local county -level illness and control institutions, and send out the complete infectious disease report card within 2 hours. After receiving the report, the county -level illness and control agencies shall immediately conduct a direct report on the Internet and make a good order of subsequent information.
If a third -party testing agency finds that the results of the test specimen are positive, they should immediately report to the county -level health administrative department where the local health and health administration is located, and the medical institution or the territorial illness and control institutions will report the relevant information within 2 hours within 2 hours. Based on its subsequent clinical diagnosis and progress, the information has been set up. If clinical manifestations occur, it should be ordered by asymptomatic infection as a confirmed case.
Suspected cases should be ordered after diagnosis or exclusion. All cases are ordered to make positive clinical severity within 24 hours according to the condition. After the case was discharged, the discharge date was reported within 24 hours. After the death of the case, fill in the death date within 24 hours.
The first case of new crown pneumonia has appeared in each county (district). Disease control institutions in the jurisdiction shall conduct direct reports within 2 hours by reporting the management information system through emergencies. Adjust and report in a timely manner based on the investigation and evaluation of the event. (2) Information reports and ordering of asymptomatic infection.
All kinds of medical and health institutions at all levels have found that the first -sieve -positive personnel of nucleic acids shall be conducted within 2 hours after the test results are issued. The network direct report, select "positive test" at the type of case type, can only be selected "asymptomatic infected" in clinical severity. The onset date is positive specimen collection time, and the diagnostic date is positive detection time. If the relevant symptoms or signs occur in the future, it is necessary to order the confirmed case within 24 hours, and the period of the onset date is the time when the occurrence of clinical symptoms or signs. After the observation of centralized isolation medicine, the medical and health institutions need to fill in the release date in the report card of the infectious disease report of the network direct reporting system within 24 hours.
(3) Reporting nucleic acid detection data.
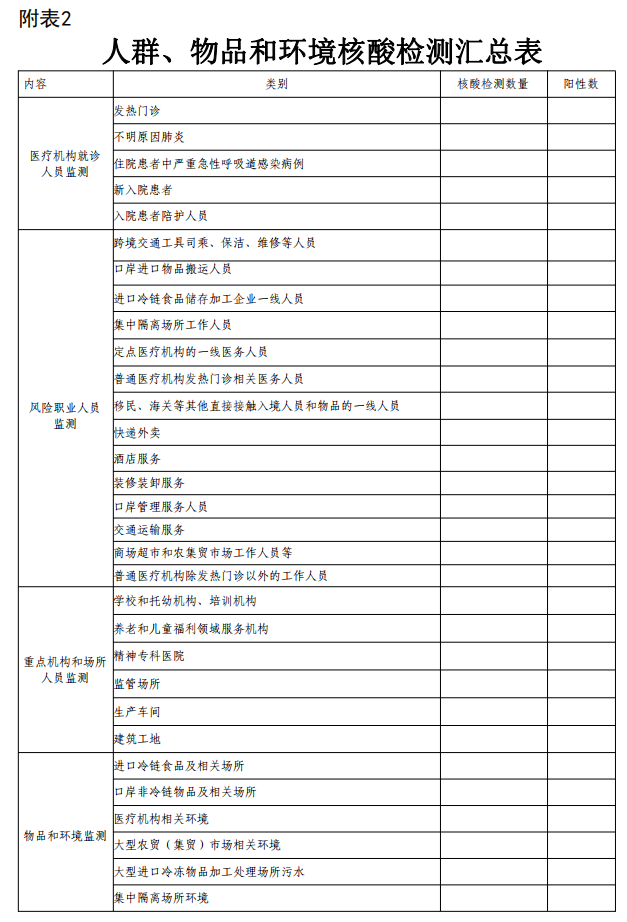
The local joint defense joint control mechanism incorporates the risk -up professional population in the area of the jurisdiction into the management of the information system, collects the number and positive number of people, items and environmental nucleic acid detection in the area in the area, and submitted the summary data (see Table 2) to the State Council Lianlian Defense Federation Federation Federation Federation Comprehensive group of control mechanism.
Seven, monitoring and management requirements
The joint prevention and control mechanisms of each province shall refine the implementation plan of the new crown pneumonia in the province in accordance with the requirements of this plan. Guide relevant departments to do a good job of information reporting to improve the timeliness, accuracy and integrity of information reports. During the monitoring process, various activities such as the collection, transportation, preservation and testing of virus strains and specimens shall comply with relevant national biological safety management regulations. All localities should establish a supervision and evaluation mechanism, urge the implementation of monitoring tasks, and evaluate the quality of monitoring work.
Affiliated table:
1. Risk -checking requirements for risk professional personnel and personnel of key institutions
2. Summary table for people, items and environmental nucleic acid testing
Source: Official Website of the National Health Commission


- END -
Shouguang Agricultural and Rural Bureau organized a \"Sanxia\" wheat harvest work meetin

#Keep in mind that you will entrust your mission to open the new bureau -in -depth...
"Urumqi-Alamu" post-cargo charter sailing

Tianshan Net News (Reporter Fan Qiongyan reported) At 12:40 on June 18, a 737-800 ...