After a lapse of half a year, the first domestic new crown special medicine for the commercialization: pricing <10,000, how to sell, who can use it?
Author:Daily Economic News Time:2022.07.08
La Dong was approved, it was listed in midsummer. In the past 7 months, the newly -dominated strains in the world were updated from Delta to Omikon, but the first domestic new crown special medicine, Tengsheng Bo medicine (HK02137, a stock price of HK $ 11, a market value of 79.50 The market influence of neutralized antibody therapy for neutralization of 100 million yuan) was unabated that shortly after the online launch conference on the online listing (July 8), the company's stock price rose rapidly, as of the closing of 11.45%.
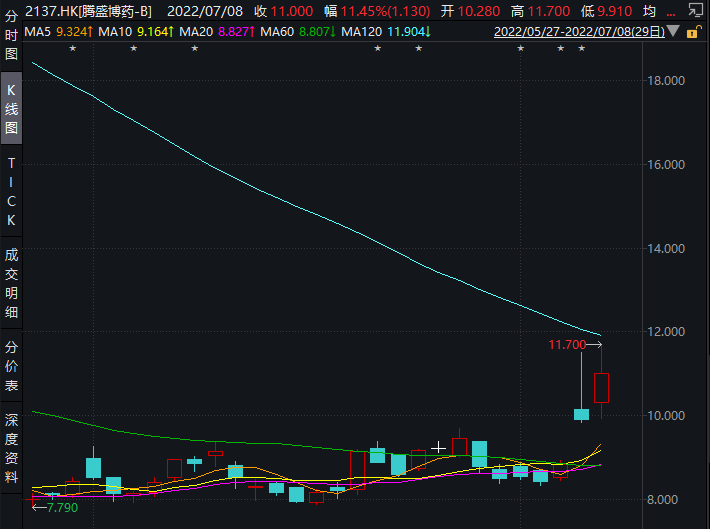
Image source: Wind screenshot
Several news is very attractive: the laboratory fake virus research shows that the therapy is still effective for suppressing the latest Omikon variants BA.4 or BA.5; The provinces and cities have incorporated it into medical insurance; the company is still exploring the new indications used to expose before/postponed ...
But there are many questions about commercialization "late": Why are therapy approval and listing for 7 months? What is the value of neutralized antibody drug therapy? How to sell, who will buy it?
At the launch conference of the drug therapy on July 8, the reporter of "Daily Economic News" received some answers from several company executives.
Double antibody+large dose, laboratory fake virus research shows that it is effective for new strains
After completing important tasks such as verifying production technology, after multiple inspections from the National Drug Administration (NMPA), Beijing Pharmaceutical Supervision Bureau, and the US Food and Drug Administration (FDA), on July 7, Teng Shengbo Pharmaceutical Pharmaceutical The announcement announced the good news of the company's long -lasting new crown and antibody Anbawei's anti -anti -anti -resistance and Lemisville solitary therapy in China's commercialization.

Photo source: Screenshot of Tengshengbo Pharmaceutical Announcement
At this time, it has been approved by the NMPA approval last year since the therapy was updated. The domestic new crown virus strain was updated to Omikon BA.5. Become the first golden stone test to test this therapy.
According to Dr. Yan Li, chief medical officer of Tengsheng Pharmaceutical, BA.5 has three great characteristics. The first is that the communication is very strong. BA.5 may become a leading new crown strain in a period of time not far.
Secondly, a mutant protein in BA.5 makes the combination of new coronary virus and alveolar epithelial cells closer, so it will not only cause infections in the upper respiratory tract, but also enter the alveoli; in addition, the strain also has immune escape ability.
According to a set of data shared by Professor Lu Hongzhou, Dean of Shenzhen Third People's Hospital, although there is currently no real virus research on BA.2.12.1 and BA.4/5, the laboratory fake virus research shows that Anbawei's anti -anti -anti -anti -anti -anti -anti -antibody therapy can still respond to BA.4 or BA.5 virus.
Specifically, the research data of a fake virus in the independent laboratory shows that the IC90 of the Ambalent monoclonal anti -anti -anti -anti -anti -anti -anti -anti -anti -anti -anti -anti -anti -anti -anti -anti -anti -anti -anti -anticipation of Omikon BA.4/5 is 16.61 μg/ml. When learning data, the blood concentration is still about 18 times the IC90 when the drug is injected.
The FDA believes that the concentration of monoclonal antibody hemorrhage can be maintained at 10 to 30 times the neutral and virus IC90 at 1 week, which is valid. Based on this, Lu Hongzhou believes that judgment can be effective for BA.4 and BA.5 for the combination therapy.
In the eyes of Dr. Zhu Qing, the senior vice president of Teng Shengbo Pharmaceutical and the head of the biopharmaceutical department, the combined therapy is "lucky", that is, every time every virus variation is changed, even if there may be some activity reduction (for example, for Austria, Austria Mik Rong BA.1, Anbawei's mate loses activity, Romi Siwei's anti -resistance is not affected, and the combination therapy is still neutral), but at least one antibody in the antibody can maintain neutrality and activity Essence
In this regard, the president of Tengshengbo Pharmaceutical and General Manager of the Greater China, Luo Yongqing, CEO of Teng Shenghua, said that this is because of the initial design of antibodies, and the virus mutations that may occur in the future have been considered. Therefore, the design is not a single antibody. It is a antibody; at the same time, considering the strength of the combination therapy, the dose of "Anbawei's monoclonal antibody (1000 mg)+Romi Siwei's Mipide (1000 mg)" is finally determined. The highest dose in neutral antibody approved in the outside world.
"Even if there are further virus mutations in the future, we can consider other methods, such as continuing to increase the dose to achieve the efficacy of (combined therapy) antiviral." Luo Yongqing said.
Picture source: Visual China
How to sell: Within 10,000 yuan, the three major dealers of China Resources, Chinese Medicine, and Pharmaceutical
At the press conference, the specific pricing of the therapy was not disclosed, but Luo Yongqing said that the final pricing of Anbawei's anti -anti -anti -combination therapy depends on multiple factors such as protein production costs and drug economy value.
At present, the company adopts the principle of autonomous pricing, which is lower than the US government's purchase price of similar neutralized antibody drugs, which is $ 1,500 to $ 2,000. Within 10,000 yuan.
It is worth noting that the combined therapy was approved by the National Health and Health Council in March 2022 and included in the "New Coronatte Pneumonic Pneumonia Diagnosis Plan (Trial Ninth Edition)". Since March 22, 2022, a number of provincial and municipal medical security bureaus have successively implemented the instructions of the notice to incorporate the combined therapy of Anbarab and Romi Siwei's anti -combination therapy into the scope of the local medical insurance fund payment.
Luo Yongqing also said that the new crown therapy drugs were paid by the government, including various situations that paid through medical insurance, finance or other methods. On the advancement path of listing across the country, Shenzhen Third People's Hospital will use it first. At the national level, the company commissioned China Resources Pharmaceutical (HK03320, HK $ 5.32, a market value of HK $ 33.423 billion), Sinopharm Holdings (HK01099, HK $ 19.72, market value of HK $ 61.539 billion), Shanghai Pharmaceutical (SH601607, stock price of 18.20 yuan, and a market value of 67.275 billion yuan). Three large dealers ensure the national coverage and quickly distribute when the epidemic occurs.
But the capacity plan is still unknown.
Luo Yongqing said that after the commercialization of the combination therapy, production plans will be formulated according to the needs of domestic and foreign markets. At present, the company appointed the trustees (CDMO) is a Yaoming creature (HK02269, the stock price of HK $ 80, and a market value of HK $ 341082 billion), but the specific output depends on the changes in the new crown epidemic in the future.
In addition, the evolution of national prevention and control policies, the pathogenesis of mutant virus, infection rate, and subsequent development have also determined the company's future production capacity reserves.
As for the commercialization of drugs, Luo Yongqing said that there are both government procurement models and traditional business models, but some commercialization methods of traditional drugs, such as Internet means, may not be completely applicable. Therefore, the company is considering and discussed with some partners to speed up the commercialization of combined therapy.
At present, Teng Shengbo has received more than 20 provinces and cities. However, the market space of the drug in the future mainly depends on the changes in epidemic prevention policies and the impact of future epidemic evolution.
"As BA.5 and BA.4 become the leading new crown virus (poison) overseas, it may also affect China. I think this will generate a need for application."
Luo Yongqing also said, "This antibody can bring performance returns is not our most important consideration." According to the press conference, Teng Shengbo drugs have invested more than 200 million US dollars before and after to ensure the research and development and commercialization of combined therapy drugs.
Picture source: Visual China

Who can use: suitable for fragile people with poor physical conditions, still exploring before/after exposure
The commercialization of neutralized antibody therapy of Teng Shengbo medicine is easy to associate with Evusheld.
Evusheld is a neutralized antibody drug, which is currently the only drug in the world that can be used for new crowns. Recently, it has passed special import approval in the advanced import approval of the international medical tourism area in Boao City, Hainan, and is suitable for new coronary viruses for adults and adolescents (age ≥ 12 years and weight ≥40kg). The price of two -shot injection of one -time injection is 13300 yuan, and medical insurance settlement cannot be used.
Luo Yongqing made a clear elaborate on the difference between Ensheng and Teng Shengbo's medicine and antibody drugs:
From the perspective of the approved indications, Eun is suitable for pre -exposure prevention. It has done a three -phase clinical trial for the treatment of treatment, but has not been approved by the FDA; Treatment.
From the perspective of the dosage of the drug, Enpara was approved at 150 mg plus 150 mg. As the new crown variants appeared, the dose increased to 300 mg plus 300 mg, a total of 600 mg. The dose of anti -combined therapy is 1 gram plus and 1 gram, a total of 2 grams, and the dose is more sufficient.
In addition, compared to Eun's muscle injection drugs, Anbawei's combination therapy is an intravenous infusion dosage form, which reaches the highest concentration in 5 hours and a half -period for several months.
从这点看,该联合疗法似乎在暴露前/后预防方面具有更大潜力,但目前该疗法仅被获批用于治疗轻型和普通型且伴有进展为重型(包括住院或死亡)高风险Adults and adolescents (12-17 years old, weight ≥40 kg) new coronary virus infection (COVID-19) patients. Among them, the adolescents (12-17 years old, weight ≥40 kg) are approved by the attachment.
From the perspective of strict and Luo Yongqing's positioning of the new crown and antibody drugs, the development of Teng Shengbo's development of the combined therapy will not stop here.
Yan Li believes that the main application scenarios of neutralized antibody drugs are divided into two. In addition to the treatment of fragile people with poor physical conditions, they can also play a preventive role for people who cannot form a protective effect through injection of vaccines.
"For high -risk professional populations, such as customs, airports, and cold chains, they can also prevent this neutralized antibody (referring to the combined therapy drugs of Teng Shengbo). Similar antibodies are It has proven to have a certain protective effect. "Yan Li said.
Luo Yongqing said that after a half -life transformation or neutralized antibody of genetic engineering transformation, the half -life can be greatly extended, and it has a protective effect of vaccine and can prevent new crowns infection and re -infection. The role of antiviral viruses also provides immune protection, which is the positioning of neutralized antibodies.
Daily Economic News
- END -
Damei Jining | Liao He Ye Shi

Figure: Jining City Photographers AssociationAuthor: Li Junhua
Hong Kong's 25th anniversary of the return of the motherland | Festive atmosphere overflows the streets of Hong Kong

On June 16, in Lidong Street, Hong Kong, the flag of the People's Republic of Chin...