The results of the i -phase Ipic 10 -stage breast cancer in my country are announced!| Asco 2022
Author:Cancer Channel of the Medical Time:2022.06.28
*For medical professionals for reading reference
Convenience and curative effects are not diminished, and oral paclitaxel treatment of advanced breast cancer is effective
According to data released by the World Health Organization International Cancer Research Agency (IARC), breast cancer has exceeded lung cancer and has become the world's number one malignant tumor in 2020. With the development of the economy, the rhythm of life, the stress of life, and the prevalence of late marriage and childbirth, the incidence of breast cancer has a steady rise.
Fortunately, with the development of new treatment drugs and the improvement of treatment strategies, the 5 -year survival rate of breast cancer has increased significantly. However, as the treatment time is prolonged, the patient's compliance and economic burden have become an important issue for the whole process of tumor. Therefore, the coexistence of development efficacy and convenience will help improve the quality of survival of patients with breast cancer and improve treatment compliance.
At the annual meeting of the American Clinical Oncology Society (ASCO) in 2022, Academician Song Erwei, Dean of the Yixian Breast Tumor Hospital of Sun Yixian Memorial Hospital of Sun Yat -sen University, Professor Yao Herui, Director of Breast Oncology and Phase II Clinical Research Center brought an assessment of oral paclitaxel at Safety and PK/PD research in patients with advanced breast cancer in my country (Abstract No.: P1104).
Screenshot of ASCO Wall News
The "Medical Circular Tumor Channel" was specially invited to the project leader, Professor Yao and Rui, to explain the relevant data and its clinical significance of the study.
Chinese oral paclitaxel research data is released!
As a common clinical chemotherapy drug, paclitaxel has a vital role in controlling the progress of tumor. However, due to its medicinal characteristics, venous preparations are the main dosage forms of paclitaxel. Patients need to come to the hospital for chemotherapy treatment. In order to improve the convenience of the treatment plan, the development of oral paclitaxel preparations is the treatment needs of tumor patients.
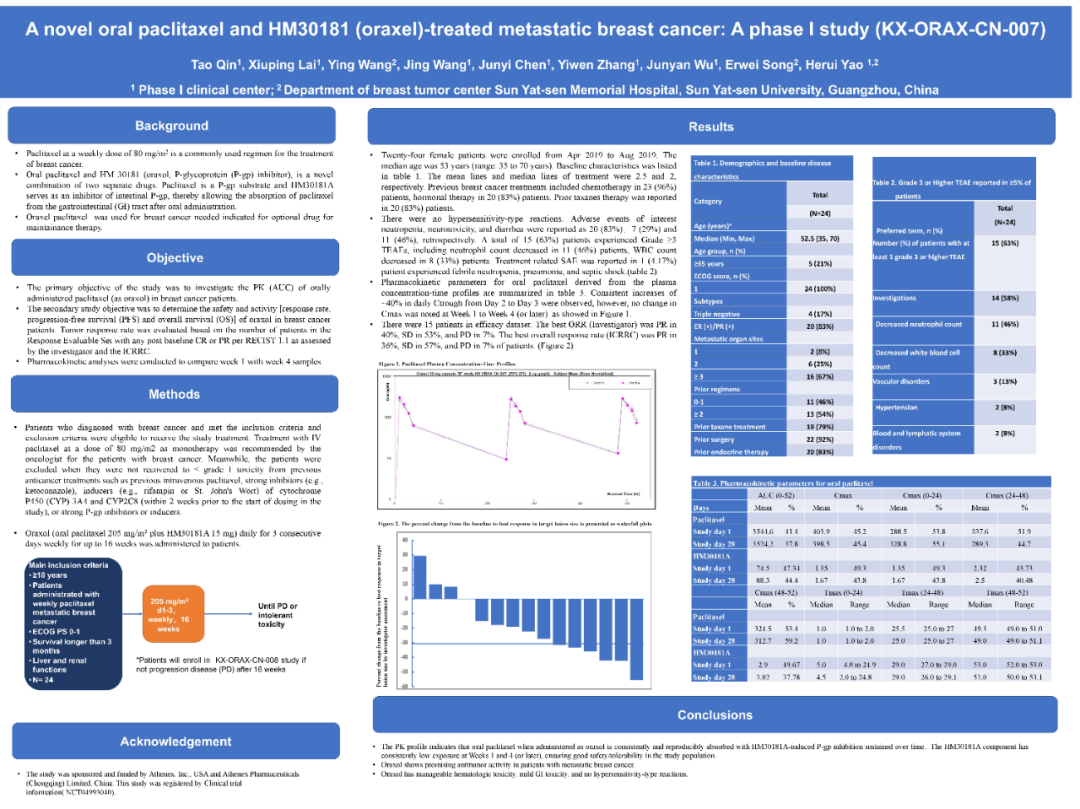
A total of 24 patients with advanced breast cancer were included in this study, all of which were taken orally at paclitaxel. On the 1st-3 days, repeated each week for 16 consecutive weeks. The median age of the whole group of patients is 53 (35-70 years old), and the number of medium and the average treatment lines is 2 and 2.5 lines, respectively. Among them, 96%received chemotherapy, 83%of the previous use of patty drugs, and 83%received endocrine therapy.
Studies have found that in the sensitive crowd of paclitaxel (N = 15), the objective relief rate (ORR) evaluated by the researcher evaluation and independent imaging evaluation committee is 40%(6/15) and 36%(5/14), respectively. Control rate (DCR) is 93%. In the study, no exposure parameters and effects and toxicity of the drug have been found.
In terms of security, the entire group did not have an allergic reaction. Any level of adverse incidents are mainly neutral granulocytes (83%), neurotoxicity (29%), and diarrhea (46%). The treatment related toxicity above level 3 is a decrease in neutral granulocytes (46%), and white blood cell decreases (decreased white blood cells (reduction of white blood cells (reduction of white blood cells (decreased (reduction 33%). One case (4.17%) of severe adverse incidents (SAE) occurred in the treatment, manifested as lack of sexual heating, pneumonia and infectious shock in granulocytes.
Convenience and efficacy are not diminished,
Oral paclitaxel treatment of advanced breast cancer results in the initial results
In the chemotherapy scheme of breast cancer, the status of paclitaxel is crucial. According to the China Clinical Oncology Society (CSCO) Guide, paclitaxel or combined targeting, immunotherapy is applied in new auxiliary therapy for breast cancer, auxiliary therapy, and endocrine therapy.
At present, clinically common clinical paclitaxel, white protein paclitaxel, and paclitaxel lipids are administered by veins. However, the vein with paclitaxel needs to be wrapped in sesame oil, which may cause superfluous reactions; in addition, long -term veins need to pay higher economic costs. Therefore, the oral paclitaxel preparations that have both effects on research and development and safety are the treatment needs that breast cancer patients have not met.
There is a certain difficulty in developing oral papletne. From the perspective of the drug mechanism, paclitaxel is a kind of P-sugar protein (P-GP) substrate, and the external pump function that is highly expressed in the gastrointestinal and tumor cells, which makes it difficult for paclitaxel to be in tumor cells. Real the concentration of effective treatment. Although the development of oral paclitaxel has a history of many years, it has not been resolved due to its oral absorption problem. There is still no oral paclitaxel drug in China.
Professor Yao Herui introduced that the test drugs for the study of paclitaxel combined with paclitaxel and P-GP pump inhibitors to achieve the absorption of the intestines that reaches the level of treatment, thereby providing a convenient and safe oral administration method. And from the results of stage I, the efficacy of oral drugs is not reduced and the safety is acceptable.
On the other hand, the condition of tumor patients in my country is not good -a considerable number of patients failed to complete chemotherapy due to the convenience of hospitalization and economic burden, affecting the prognosis of these patients. If patients can use oral chemotherapy drugs to complete the chemotherapy treatment at home, while improving the quality of life, they can benefit from complete and standardized chemotherapy treatment.
At present, effective and convenient oral chemotherapy drugs such as Koriebin, Changchun Ruibin have been clinically "Dajian" and effectively extended the survival of patients. If oral paclitaxel can be successfully developed in the end, more patients with breast cancer will benefit from standardized treatment.
Professor Yao Herui said that the study, as a phase I study of the domestic oral paclitaxel, not only confirmed that oral paclitaxel has good curative effect and safety for advanced breast cancer in China, but also provides strong support for subsequent phase II/III research.
The first release of this article: the medical world tumor channel
Author of this article: Yao Herui, Qin Tao
- END -
The person in charge of the Hunan Provincial Department of Commerce came to Changde High -tech Zone to investigate and investigate

On July 1, Guo Ning, a member of the Party Group and Deputy Director of the Provin...
Huiyang Meteorological Observatory lifted the yellow warning of heavy rain [III level/heavier]
Huiyang District Meteorological Observatory June 08th at 19:28 to relieve the yellow warning of Huiyang District