Mao Li joined!"Low -key" Sisheng Pharmaceuticals hidden in innovation "gold mines"
Author:Kenji Bureau Time:2022.09.27
In 2010, Belief Schimibao published a milestone paper in the New England Medical Magazine.
The clinical trial data disclosed by this paper proves the potential of immunosuppressants in the treatment of tumors. These drugs allow the human T cells to correctly identify cancer cells and kill it, providing new ideas for the treatment of tumors.
The scientist of the US pharmaceutical company Moshadong saw this paper and immediately decided: restarted the research project that has been put on hold for 7 years, and strive to carry out clinical clinical in early 2011.
This project in Murisha was a well-known PD-1 inhibitor later, commonly known as "K medicine" Paborzab. Meridon saw that PD-1 can play a basic position in tumor treatment.
In the past, people's treatment of cancer was mainly used to use chemotherapy or targeted drugs to directly hit the cancer cells itself. But immune drugs have opened another new idea, which can produce a lot of treatment around it. Many pharmaceutical companies in China continue to develop PD-1/PD-L1.
In the pharmaceutical industry, finding a "basic drug" and providing new ideas for disease treatment is a scientific research progress that many scientists dream of.
In June of this year, Mao Li, vice president of Sisheng Pharmaceutical Industry, and general manager of R & D and chief medical officer, was to find China's own basic drugs and tapped the "gold mines" one by one, and took out a unique innovation path. Come.
Digger more innovative "gold mines"
The SARS epidemic 20 years ago made people remember.
SARS comes fast and fast, and scientists have disappeared without any time to study it. The treatment plan at that time was far from being so calm when dealing with the new crown today, which was also one of the regrets in the academic world. At that time, a lot of medicines were the product of Sesheng Pharmaceutical, "Ridama", which was new.
The thymus is new belongs to thymptopptide drugs and is an immune regulator. In 1996, SCICLONE US, the predecessor of Sesheng Pharmaceutical, pushed this drug to the Chinese market. In the 2003 SARS epidemic, it was famous for being widely used for resistance.
However, for many years, Ridamian's positioning has not been effectively broken. The outside world has stayed in the auxiliary medication by the stereotypes of it, but its understanding of its progress in the field of tumors is limited.
If a drug can provide more solutions for the clinical treatment of multiple diseases, it is called "basic drugs". In Mao Li's view, Ricianxian is such a variety with the potential of "basic drugs". He said: "When I came to Cai Sheng Pharmaceutical, the first thing was to face the problem of re -positioning Nitthadianxian, and explore the path of the cornerstone products in the field of immunotherapy in the world."
Mao Li has more than 35 years of oncology clinical practice, basic research and leadership experience. It has created a global lung cancer research center across its three major business areas and is a well -known expert in the field of tumor. In 2018, Maoli returned to China, joined Beida Pharmaceutical, and successfully led Beda's second innovative drugs and ALK inhibitors Ensaidinib was approved to go public.
Such experience made Mao Li get familiar with China's drug innovation environment. He frankly said: "ME-TOO, ME-Better's drugs are important, but I am more interested in putting my limited energy into more important innovations. And China is currently in China. The homogeneity tendency of innovative drugs will definitely be corrected. "
After leaving Beida, Maoli has shortly worked in many companies. In his own words, "I want to see where I can help the company to do real innovation." In the end, he came to Sesheng Pharmaceutical, "For me, Sheng is a suitable soil."

In addition to Ridama, there are actually many varieties that enter commercialization, including the "selection" for the treatment of early breast cancer and bone protection, and the metastasis of malignant tumor bone. Matkazole's cavity "Nomo Ke" and so on. In the research lineup, it is even more powerful. For example, a targeted targeted genetic regulatory small molecule RRX-001, neurological maternal cytoma immunotherapy drugs with the potential of First-in-CLASS potential.
Mao Li pointed out that there are two types of original, one is to develop completely new targets and huge potential molecules; the other is a new application of mature drugs. In the context of the current overall downturn in China's innovative pharmaceutical industry and the difficulty of new drugs, if these potential and mature varieties can be effectively developed, they will be a "gold mine".
Shengsheng also has the ability to tap these two "gold mines".
"It's hard to classify", but there is a clear direction
If more common classification methods, pharmaceutical companies can be roughly divided into large multinational pharmaceutical companies, local private pharmaceutical companies, innovative pharmaceutical companies, small and medium -sized foreign companies, etc.
"Sesheng Pharmaceutical has always been low -key, and it is difficult to return to which category. It has its own characteristics." This is Mao Li's evaluation of Shengsheng.
At present, most of the License-in varieties in Sesheng Pharmaceutical Pipeline. For a period of time, License-in is often labeled with "insufficient research and development capabilities", but Mao Li does not think so.
Mao Li introduced: "Johnson & Johnson has more than 10,000 R & D teams worldwide, but not all varieties are completely developed independently. For example, the heavy variety Ibetinib is not the original of Johnson & Johnson. I am very recognized by Johnson & Johnson: The company has so many scientists Finding the right introduction project for the company is one of their important duties. "
License-in is not a way that can be successful casually. In fact, the comprehensive ability of the introducer is very tested. The vision of choosing a product is particularly important. Mao Li believes that on the one hand, it is necessary to grasp the cutting-edge drugs, for example, not long ago to complete the third-line lung cancer three-line and above treatment of the international multi-center III clinical trials of China ’s first RRX-001 for Chinese patients. A variety of action mechanisms such as genetic targeting and RONS generation are no precedent species worldwide; on the other hand, they value later varieties and even mature varieties. A simple bridge test can quickly realize commercial value. These two categories of drugs are important directions for the introduction of varieties in the future. These R & D layouts are aimed at clinical "unsatisfactory treatment needs", which is a direction that clearly gives "accelerated listing" in the registration of national drug registration. The goal of Shengsheng is very clear.
At the same time, Maoli hopes to use the momentum of mature varieties and potential pipelines to enhance the strength of basic research in the field of tumors and severe infections. Basic research will not be effective in the short term, but if a company wants to achieve sustainable development, it is necessary to strengthen self -research ability.
During the development of Benda Pharmaceutical Development Enshaidinib, Maoli led the team to build Beda's clinical development structure. He attaches great importance to the ability of clinical development and is determined to make Sheng's clinical development ability into an industry benchmark. Mao Li said: "I hope that through the project of RRX-001, a 'iron army' forging clinical development will be forged." These accumulated experience of clinical development can also be quickly transformed into other varieties.

In the future development model, Maoli considers Casual to continue to actively cooperate with domestic and foreign academic institutions and innovative enterprises. , Hatch more cutting -edge products. At the appropriate time, it will also consider acquiring R & D companies or platforms that can be highly complementary with Shengsheng.
Innovative pharmaceutical industry
The current Sesheng Pharmaceuticals has stable returns, and there are also a combination of potential pipelines. It should be said to have the ability to attack.
However, Shengsheng did not blindly expand his business boundary, but he was cautious and low -key along the direction he was identified. Faced with the stage of Chinese pharmaceutical innovation, Mao Li had a very sober understanding.
In the past ten years, the Chinese pharmaceutical industry has gradually followed up from imitation to the main use, and some independent innovation products have come out. Chinese scientists and clinicians are much more confident than before. Mao Li believes that the real independent research and development will definitely go out from China in the future and get international recognition.
But in this process, there will be a relatively large shuffle process. At present, some innovative pharmaceutical companies have experienced the problem of capital chain. In the future, industry advantages will be concentrated in some enterprises, and the tendency to develop homogeneity will be corrected. During the shuffle stage, it is critical to maintain a stable cash flow.
"Enterprises such as Sesheng who have a good cash flow will survive." Mao Li is confident that Sai Sheng can seize the opportunity, make research and development, and continue to advance in the follow -up pipeline and become an industry winner. "That's why I am willing to join Shengsheng."
Shengsheng will still maintain the "light asset" operation model, and use more funds to transport "grain grass" to the R & D side. "Many enterprises have grown up to the capital, and they go to the circle to cover the factory building without doing anything. In fact, the investment in this investment is very low." Mao Li believes that the "full chain of the industry in the industry must be controlled by me must be controlled" Turn.
On the other hand, all companies attach great importance to the commercialization capabilities. Industry people have long been consensus, lacking the experience of commercialization teams and commercialization. It is a "shortcoming" that many innovative pharmaceutical companies in China need to overcome. The two indicators can explain the level of commercialization of Shengsheng: the sales market share of the main product Ridianxian has continued to increase in recent years. At the same time, the sales cost rate has long been lower than the industry average. Mao Li pointed out that strong commercialization capabilities will be able to accelerate the return of innovation and help the international layout of contestants.
In 2030, he entered the top 50 global pharmaceutical medicines, forming a pattern of about 80%of the market in China and 20%overseas. This is the long -term target of Shengsheng. Mao Li said: "The board of Shengsheng is fully authorized and has a lot of constructive opinions on the company's development prospects. The company is very recognized by the direction and internationalization of development strategy."
Mao Li hopes that the innovative development path of Sesheng Pharmaceuticals can be used to the entire industry. "Now the information is flat. The global scientific community's judgment and understanding of new things is already on the same horizontal line. Not to mention that the Chinese are so diligent that Chinese pharmaceutical companies have no reason to achieve the results of innovation."
Writing | Jianxian Bureau

Edit | Jiang Yun Jia Ting
Operation | Twenty -thirty
Illustration | Visual China
####
- END -
Why can the insulation cup vacuum layer be insulated?What is the difference between products such as insulation cotton?
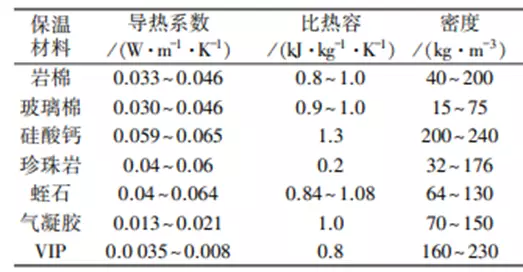
answer:The physical principles have a different way to return. Both the vacuum lay...
Small positions, big services, small units and big happiness!Wutian Street Promote the Construction of "Sharing Club · Happiness"

The children's picture book sharing session of the Lakeside Book House made me lik...