2022 ESMO Professor Xia Wen: Oral SERD research explores the twists and turns, but it is still worth looking forward to
Author:Cancer Channel of the Medical Time:2022.09.25
*For medical professionals for reading reference
What are the latest research progress of oral SERD? What are the clinical revelation behind it? Come and check in.
For HR+/HER2-breast cancer, endocrine therapy is indispensable. Common drugs include selective estrogen receptor (SERM, such as him, such as Monifen), selective estrogen receptor (SERD, such as Flivius group), aromatase inhibitors (AI), etc. From the perspective of the mechanism of action, AI can play an anti -tumor effect by inhibiting estrogen generating, and both Moqifen and Flivis can block estrogen receptors (ER), but the Fluvis group is currently the only one that can both be both able Combining, blocking, and degradable ER endocrine drugs, have a dual effect of inhibitory and active receptors, and the mechanism is better than SERM. Not only that, because ESR1 mutations can cause ER to depend on estrogen and abnormally active, it is an important reason for AI drug resistance, and those carrying ESR1 mutations are still relatively sensitive to the treatment of fluorozi group [1,2].
The fluoros group has obvious advantages and is an important choice for breast cancer endocrine therapy. However, because the injection type may limit the scope of the use of such therapies, this prompting the industry to start finding a more convenient and higher new drug format. Oral SERD came into being and became an important area for many pharmaceutical companies to compete. In 2022, the European Cancer Internal Science Society (ESMO) conference has four studies of four oral SERDs. The "Medical Circular Cancer Channel" specially invited Professor Xia Wen of the Sun Yat -sen University Tumor Prevention Center to interpret the latest research progress of oral SERD.
Oral SERD's latest research progress
At present, a number of oral SERDs have announced the data of Phase I-II research data. This article focuses on the ESMO conference that the latest research progress of the latest research progress of the ESMO conference, the latest research progress of the oral Serd Elacestrant, Roche's Giredestrant, and the Amcenestrant of Selofe Inventory and analysis to readers.
AMCENESTRANT's research progress
Based on AMCENSTRANT's AMEERA series research, it is full of twists and turns. In the I/II phase AMEERA-1 study of amcenestrant, AMCENSTRANT combined with Bai Cyri, the ORR confirmed by patients with post menopausal female breast cancer was 5/46 (10.9 %). The overall clinical benefit rate (CBR) is 13/46 (28.3%) [3]. Although from the perspective of efficacy data, AMCENESTRANT combined with Bercyli treatment ER+/HER2-postmenopausal breast cancer shows preliminary clinical benefits, but in March this year, Sanofi announced that its Amera-3 research failed to reach its failure to reach The main end point PFS [4], this ESMO conference announced detailed data.
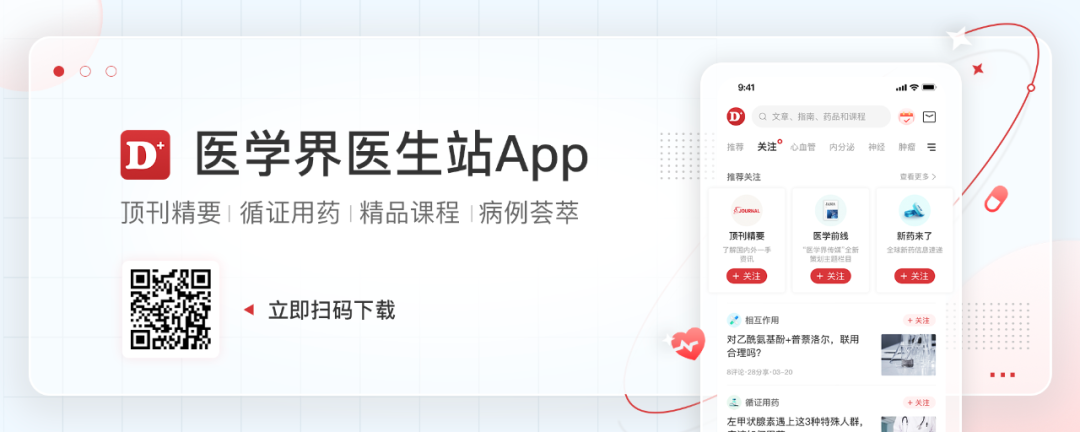
Phase II AMEERA-3 Research aims to evaluate AMCENESTRANT single medicine treatment compared to the single-drug endocrine therapy selected by the doctor (TPC, including Flui Siqun, aromatic enzyme inhibitors, or he Moqifen) in the past ER+/HER2- Local advanced or metastatic breast cancer patients have curative effect and safety. The results released by ESMO in 2022 showed [5], AMEERA-3's research failed to reach the main end point, and the PFS value evaluated by each independent center review (ICR) was similar (3.6 months vs 3.7 months, HR = 1.051, 95, 95 % CI: 0.789-1.4; P = 0.6437). The results evaluated by researchers and the pre -set sub -groups (including ESR1 mutations) are consistent with the aforementioned results. OS is also similar in value (data is not yet mature). The results showed that AMCENESTRANT did not bring survival benefits for ER+/HER2-advanced breast cancer for endocrine therapy.
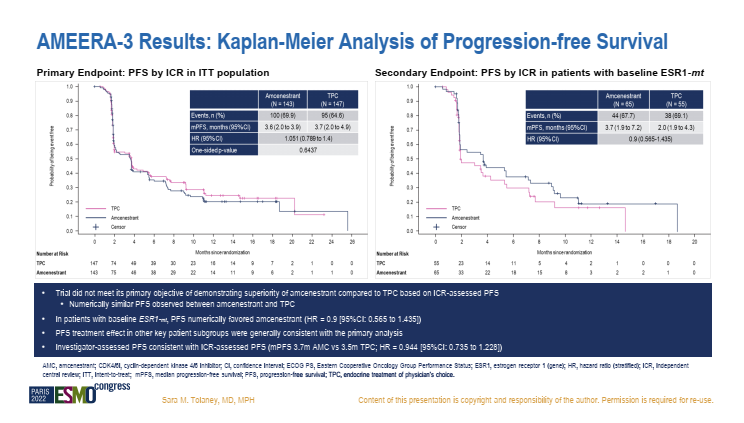
Figure 1. PFS result of the research
Not only that, AMCENESTRANT's Phase III-5 studies also ended in failure, and based on the results of the research, in August this year, Selofe officially announced that it would stop all global development plans of AMCENSTRANT [3]. The failure of Amcenestrant undoubtedly sounded the alarm for oral research and development.
Research Progress of GiredESTRANT
The results of GiredESTRANT's previously published research shows that in advanced breast cancer, its single medicine or combined therapy has good anti -tumor activity and tolerance. For example, the IB stage of GiredESTRANT shows [6]. For patients with local advanced or metastatic ER+/HER2-breast cancer patients in the past, Giredestrant shows good results, and patients with ESR1 mutations are also effective. However, the research results of GiredESTRANT's II Acerara BC have encountered setbacks. As early as April, Roche announced that the study failed to improve the main research end of PFS [7], and its detailed data was announced at the ESMO conference.
II Phase Acelera BC Studies are incorporated into advanced ER+/HER2-breast cancer that has been previously received in the treatment (must include endocrine therapy), and explores the endocrine therapy (PCET, Flui Si group or aromathered enzyme inhibitor selected by the Giredestrant compared to the doctor. ) The efficacy and safety of second or third -line therapy. The data released this time showed that [8], the CBR of the Giredestrant and PCET groups was 31.8%and 21.1%, respectively; ORR was 12.6%and 7.2%, respectively. The PFS evaluated by the researchers was 5.6 months and 5.4 months, and HR = 0.81 (95% CI: 0.60, 1.10, P = 0.18). OS data is not mature yet. The research results show that the Acelera BC has not reached the main endpoint of INV-PFS, and Giredestrant only shows the improvement of the PCET value. This seems to be a blow to the emerging oral SERD exploration. But CBR and ORR values are higher, and it is worth noting that PFS benefits in patients with ESR1 mutations are more significant. It seems that GiredESTRANT seems to have prospects in patients with ESR1 mutations, but more sample volume research is required to verify. Fig

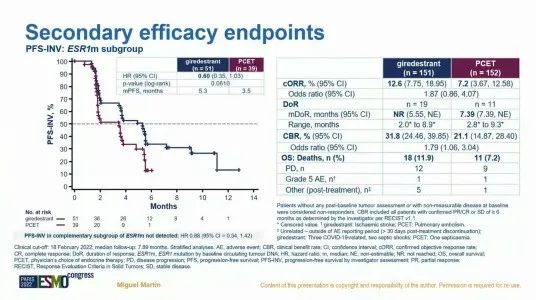
Although the research exploration of GiredESTRANT in advanced breast cancer is not satisfactory, its performance in the field of early breast cancer therapy is gratifying. ER+/HER2-early-stage breast cancer neo-assisted treatment and safety. The final analysis results show that [9], the Giredestrant combined with the Berryi group compared to the Anustezozozozozozoli combined with the pyrite group, observed the inhibitory effect on KI67 during the operation, and achieved a greater degree of complete cell cycle during surgery. Block (CCCA; Ki67 ≤2.7%). But the ORRs of the two groups are similar (50% vs 49%). The analysis of the sub -group of the biomarkers of the study [10] this time announced in the giredestrant combined group treatment The level of 2 weeks of Ki67 is more significant. And the ER and PGR protein also declined significantly.
Figure 3. Analysis of biomarkers studied by Coopra studies
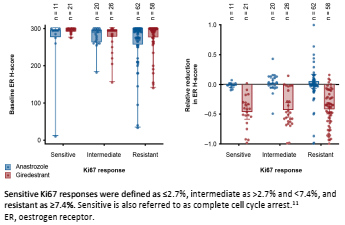
Not only that, GiredESTRANT also conducted positive exploration in the field of early breast cancer auxiliary treatment. LIDERA is a stage of III studies compared with Giredestrant and endocrine single-drug-assisted therapy in ER +/HER2-early breast cancer patients [11]. It is currently underway.
Research Progress of Elacestrant
Although the two oral SERD drugs have failed, it does not mean that oral SERD will always be sluggish. The appearance of Elacestrant brings hope for the development of oral SERD drugs. In its III phase EMERALD study, patients who have previously received 1 or 2 endocrine therapy have been included, and all patients have received CDK4/6 inhibitors. Randomly distributed to ELACESTRANT and Standard Endocrine therapy (SOC, including Flivids, Nenatzole, Calibazole or Iseam). The main end of the study is the PFS of the overall population and the patients with ESR1 mutations. The results released by SABCS in 2021 showed [12], which reached two main endpoints. Among the general population, the median PFS of the Elacestrant group and the SOC group are 2.79 months and 1.91 months, respectively (HR = 0.697 [95% CI: 0.552 ~ 0.88]; P = 0.0018). Among the ESR1 mutants, the median PFS of the two groups were 3.78 months and 1.87 months, respectively (HR = 0.546 [95% CI: 0.387 ~ 0.768]; P = 0.0005). In 2022, the ESMO conference announced the results of the Asian group analysis of ELACESTRANT compared to different endocrine therapy drugs in the study [13]. Benefit. Further verification the potential of ELACESTRANT single drugs in the second or third-line treatment of ER+/HER2-advanced/metastatic breast cancer.
Figure 4. PFS result of EMERALD research

Based on the results of this research, the FDA has been awarded ELACESTRANT priority examination in August this year [14], which means that Elacestrant is expected to become the first oral SERD drug to be approved for listing. At the same time, Elacestrant's success has re -established researchers' confidence in continuing to take oral SERD.
Expert Reviews
Flui Siqun is a SERD drug with muscle injection. In the past, a series of studies fully confirmed the important value of its HR+advanced breast cancer. At present, Fluowis Group has become HR+/HER2-advanced breast cancer endocrine therapy therapy One of the cornerstone. As early as the beginning of the Flui Siqun group, he tried to develop a SERD oral dosage form. The new oral SERD drugs are modified by chemical structure to improve the solubility and polarity of the ER degradation compound, and after multiple update iterations to further improve biological utilization [15]. And it is more convenient in medication, which can effectively increase patient's treatment compliance. However, while improving biological utilization and convenient medication, whether its efficacy is better than Fluowis Group is a question of being exploring. In addition, the ESR1 mutation is the difficulty of endocrine therapy. Compared with the Flui Si group, it is also a hot spot for clinical attention in the future. Through this year's ESMO research data, we have also obtained some clinical revelation.
First of all, the efficacy of oral SERD and fluorosami groups is PK, is it good or bad?
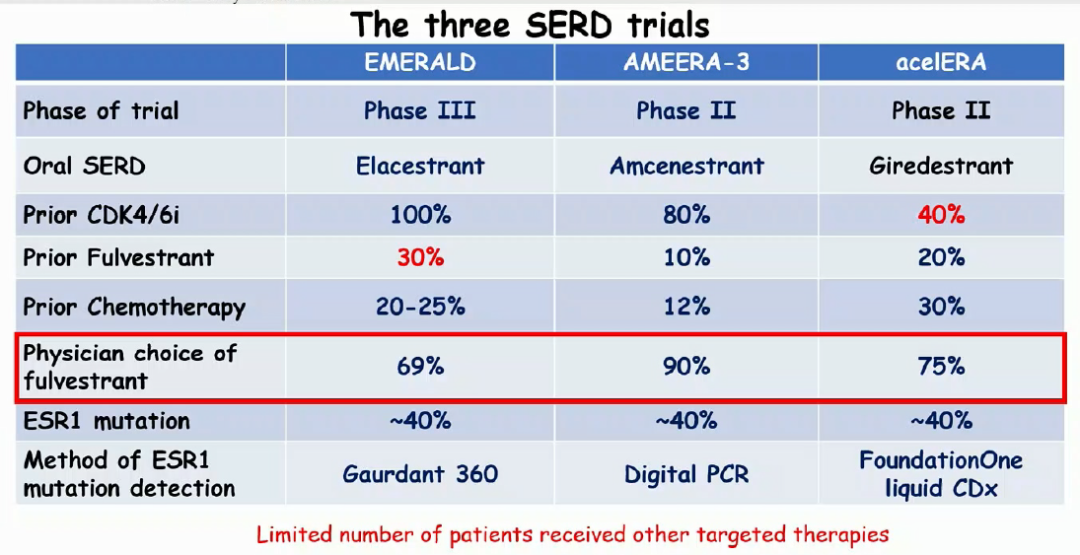
For patients with HR+/HER2-advanced breast cancer, currently fluoros single medicine or fluoros group combined with CDK4/6 inhibitors are commonly used for advanced standard treatment options. For the above three oral SERD drugs, from the perspective of the research group group, EMERALD research (100%) and AMEERA-3 research (80%) are included in patients with a higher proportion of CDK4/6 inhibitors. The Acelera BC research entered the group only 40%of the CDK4/6 inhibitors. In terms of comparison, the enrolled groups of Emerald's research group may be one of the reasons why PFS is shorter. In addition, it is worth noting that the proportion of patients with the patients selected by Chinese doctors in the above clinical research control group is high, and the proportion of patients with Fluowis Group is relatively high. The three studies are 69%, 90%, and 75%. Fluowi group. But at present, only EMERALD has achieved statistical differences in the general population, and this time ESMO analyzes the efficacy of comparison of fluoros, consistent with the entire population. The other two oral SERDs did not see a significant difference in curative effect. Therefore, except for the advantages of the dosage form, there is no additional benefits in the curative effect. Of course, more data are required to confirm it.
Figure 5. 3 Oral SERD Drug Clinical Studies Comparison
Secondly, can oral Serd overcome endocrine therapy for ESR1 mutations?
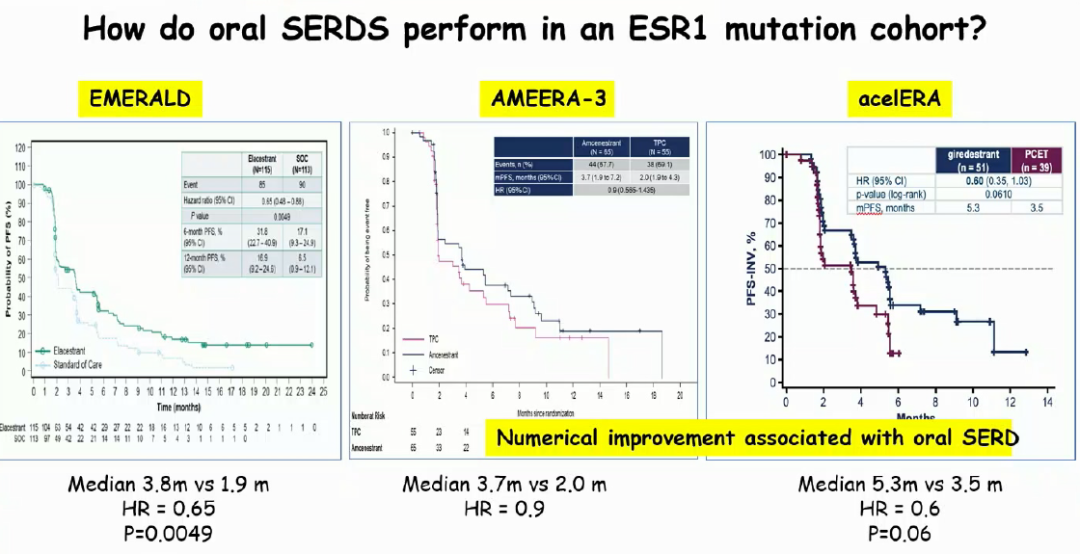
Clinical and pre-clinical research [16-18] shows that ESR1 mutations are mainly enabled in receiving endocrine therapy, especially after AI treatment, recurrence and metastasis of breast cancer, and the proportion of patients with endocrine resistance can reach 30-40%[ 19]. And some researchers have found that all patients carrying ESR1 mutations have previously received AI treatment [20]. The retrospective analysis of Sofea and Paloma-3 test [22] shows that after AI treatment, CTDNA detection ESR1 mutations can be used, and ESR1 mutants can still be relatively sensitive to the treatment of fluoros group. It also confirms the prognosis advantage of Flui Si group in patients with ESR1 mutations [23]. Therefore, for AI drug -resistant ESR1 mutant patients, you can try to choose the treatment plan for fluorophye groups. To better overcome the resistance caused by ESR1 mutations is one of the developed motors of the oral SERD, but what is the specific effect?
Judging from the research itself, for the ESR1 mutant Asian group, only ELACESTRANT has a significant benefit compared to the endocrine therapy drugs chosen by the doctor. 0.65, P = 0.0049). The other two oral SERD PFS have only the advantages of value, of which amcenestrant HR = 0.9, the research has also been suspended; and Giredestrant's PFS HR = 0.6, P = 0.06, considering the problem of samples, although the statistical difference is not obtained, although the statistical difference is not obtained, it has not obtained statistical differences. However, the potential needs to be further data verification. For patients with ESR1 mutations, the efficacy differences between different drugs may also be large, and we look forward to more data to discuss.
Fig
Summary and outlook
Judging from the above -mentioned research progress, although oral SERD's research and development is not smooth, the road to exploration is far from over. For example, Giredestrant and Elacestrant are still continuing for subsequent clinical layout. The field has achieved preliminary results or is being deployed. All these show that the application prospects of oral SERD are still worth looking forward to. I hope that more research data can be disclosed as soon as possible and further enrich the endocrine therapy strategy of HR+/HER2-breast cancer.
Expert Introduction
Xiawen
Master of Master of the Internal Medicine of the Internal Medicine of Sun Yat -sen University on the Cancer Prevention and Treatment Center

Dr. Beijing Union Medical College (Department of Medicine, Tsinghua University)
The 6th "Yangcheng Youth Good Doctor"
Standing Committee Member of the Chinese Research Hospital Society of Breast Cancer
Standing Committee Member of the Guangdong Provincial Breast Diseases Society
Member of the Youth Committee of the Breast Cancer Professional Committee of the Guangdong Provincial Anti -Cancer Association of the Guangdong Province
Member of the Breast Cancer Professional Committee of Chest Tumor Prevention and Treatment Society in Guangdong Province
JCO Chinese Editorial Committee American Case Western Reserve University affiliated University Hospital, Cleveland and French Pitie-SALPETRERERe, Paris exchanges.
CN-103093 Expiration date: 2023-9-22
Disclaimer: This material is supported by Astrikon, for reference for medical and health professionals
references
[1]. In summary of Wang Jiayu, the research progress of Xu Binghe's review. Fluvis group for postmenopausal hormone receptor positive breast cancer [J]. China Cancer Magazine, 2016, 26 (5): 5.
[2]. Li Yanjun, Xu Binghe. The research progress of breast cancer aromatic enzyme inhibitors [J]. China Cancer Magazine, 2021, 31 (2): 9.
[3].Bardia, A.,Chandarlapaty, S., Linden, H.M.et al.AMEERA-1 phase 1/2 study of amcenestrant, SAR439859, in postmenopausal women with ER-positive/HER2-negative advanced breast cancer.Nat Commun13 , 4116 (2022).
[4] .https: //www.sanofi.com/en/media-ileom/press-reases/2022
[5].TolaneySM, Chan A, Petrakova K , et al. AMEERA-3, a phase II study of amcenestrant (AMC) versus endocrine treatment of physician's choice (TPC) in patients (pts) with endocrine-resistant ER+/HER2− Advanced Breast Cancer (ABC). 2022 ESMO. 212MO.
[6].Komal L. Jhaveri, Valentina Boni, Joohyuk Sohn, et al. Safety and activity of single-agent giredestrant (GDC-9545) from a phase Ia/b study in patients (pts) with estrogen receptor-positive (ER+ ), Her2-Negative Locally Advanced/Metastatic Breast Cancer (LA/MBC). 10.1200/JCO.2021.39.15_suppl.1017 Journal onCology 39, NO. 15_SUPPL (MAY 20, 2021) 10.7-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17-17 10.7-17-17 10.7-17 10.7-17, 1021) 10.7-17 10.7-17 10.7-17 10.7-17-17 10.
[7] .Roche's Oral Serd Giredestrant Fails Breast Cancer TRIAL-
[8].Jimenez MM, Lim E, Gregor MC,et al.Giredestrant (GDC-9545) vs physician choice of endocrine monotherapy (PCET) in patients (pts) with ER+, HER2– locally advanced/metastatic breast cancer (LA/ MBC): Primary Analysis of the Phase 2, Randomised, Open-Label Acelera BC Study.2022 ESMO.211Mo.
[9].Peter A. Fasching, Aditya Bardia, Vanesa Quiroga, et al. Neoadjuvant giredestrant (GDC-9545) plus palbociclib (P) versus anastrozole (A) plus P in postmenopausal women with estrogen receptor–positive, HER2-negative, untreated early breast cancer (ER+/HER2– eBC): Final analysis of the randomized, open-label, international phase 2 coopERA BC study.2022ASCO. 589.[10].Bardia A, Fernando TM, Fasching PA,et al. Neoadjuvant giredestrant (GDC-9545) + palbociclib (P) vs anastrozole (A) + P in postmenopausal women with oestrogen receptor-positive, HER2-negative, untreated early breast cancer (ER+/HER2– eBC): Biomarker subgroup analysis of the randomised, Phase II Coopera BC Study.2022 ESMO. 144p.
[11] .ClinicalTribis.gov Identifier: nCT04961996 2021 SABCS. OT2-11-09.
[12] .POSITIVE EMERALD TRIAL Results for Elacestrant Presented at San Antonio Breast Cancer Symposium 2021
[13].Aftimos PG,Cortés J,Bidard FC ,et al. Elacestrant vs fulvestrant or aromatase inhibitor (AI) in phase III trial evaluating elacestrant, an oral selective estrogen receptor degrader (SERD), vs standard of care (SOC) endocrine Monotherapy For E er+/Her2-Advanced/Metastatic Breast Cancer (MBC): Subgroup Analysis from EMERALD.2022 ESMO.220p.
[14 ].menarini Group ’s ELACESTRANT GRANTED Priority Review by the U.S. FDA FOR PATIENTS with ER+/HER2-Advanced Orast Cancer
[15].Lloyd MR, Wander SA, Hamilton E, Razavi P, Bardia A. Next-generation selective estrogen receptor degraders and other novel endocrine therapies for management of metastatic hormone receptor-positive breast cancer: current and emerging role. Ther Adv Med Oncol. 2022 Jul 30; 14: 17588359221113694.
[16]. Dustin D, Gu g, Fuqua S. ESR1 Mutations in Breast Cancer [j]. Cancer, 2019, 125 (21): 3714-3728. Al. ESR1 Ligand-Binding Domain Mutations in Hormone-Resistant Breast Cancer [j]. Nat Genet, 2013, 45 (12): 1439-1445.
[18] .zhu W, Ren C, Wang Y, Et Al. Prevalence of ESR1 Mutation in Chinese Er-POSITIVE BREAST CARANCER [J]. Onco Targets THER, 2020, 13: 615-621.
[19].Liao H, Huang W, Pei W, Li H. Detection of ESR1 Mutations Based on Liquid Biopsy in Estrogen Receptor-Positive Metastatic Breast Cancer: Clinical Impacts and Prospects [J]. Front Oncol. 2020;10:587671.
[20].FREITAG C E, MEI P, WEI L, et al. ESR1 genetic alterations and their association with clinicopathologic characteristics in advanced breast cancer: a single academic institution experience[J]. Hum Pathol, 2021, 107: 80-86.
[21] .zhang k, hong r, xu f, et al. CLINICAL VALUE of Circulating ESR1 Mutations for Patients with Metastatic Breast Cancer: A Meta-Analysis [J]. Cancer Manag Res, 2018, 10: 2573-25888888888888888888888888888888888888888888888888888888888888888.
[22] .fribbens C, O'Leary B, Kilburn L, et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor-Positive Advanced Breast Cancer [J]. Then, then, then
[23].TURNER N C, SWIFT C, KILBURN L, et al. ESR1 mutation and overall survival on fulvestrant versus exemestane in advanced hormone receptor-positive breast cancer: a combined analysis of the phase Ⅲ SoFEA and EFECT trials[J]. Clin Cancer Res, 2020, 26 (19): 5172-5177.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
Strong precipitation is coming!Pay attention to these areas in the north | Expert Interpretation

Shaanxi, Shanxi, Hebei, Shandong, heavy rain, Sichuan Basin, Huanghuai, Jianghuai ...
Deepen the construction of Yongxing Town, Yongxing District, Pianshan District, Suining City in the country.
In order to deepen the construction of civilized cities across the country and create a cleaner, tidy, civilized and harmonious and livable environment, Yongxing Town fully integrates resources, grasp