2022ESMO Professor Liu Liping: DATA research provides new evidence -based evidence for extending assistance endocrine therapy for high -risk people
Author:Cancer Channel of the Medical Time:2022.09.24
*For medical professionals for reading reference
Extend the beneficiated analysis of the auxiliary endocrine therapy in the treatment of early breast cancer treatment of hormone receptors.
For early hormone receptors (HR) positive breast cancer, it has an important role in assisting endocrine therapy (standard time for 5 years [1]). The latest research progress has shown that extending the length of therapy of assistant endocrine therapy may optimize the treatment efficacy of some patients. In recent years, the strategy of extending assisted endocrine therapy in recent years has gradually been accepted and adopted by clinicians. However, there are still many controversy on which patients need to extend assisted endocrine therapy and the best time to extend assisted endocrine therapy [2]. In 2022, the European Cancer Internal Science Association (ESMO) announced the update results (Abstract number: 133o) [3], which provides ideas for the auxiliary endocrine therapy strategy of patients with early HR positive breast cancer. At the same time, a real -world study recently published has also explored the beneficiaries of extended auxiliary endocrine therapy and prognosis. [4], the medical circles invited Professor Liu Liping of Hunan Cancer Hospital for interpretation of relevant research progress.
The final analysis results of DATA Research suggest that HR positive early breast cancer extension auxiliary endocrine therapy after menopause is significant
Data research design
DATA research is a forward -looking, random, open -label phase III clinical trial. From June 2006 to August 2009, 1912 patients with a menopause of 79 centers in the Netherlands were admitted to patients with early HR positive breast cancer. Patients who comply with the enrollment standards After taking him for 2-3 years auxiliary treatment, if there is no recurrence, it is randomly assigned to an Annotrazole for 3 years or 6 years. The layered factors include: lymph nodes, HR status, HER2 status, and the continuous treatment time of his Tam (TAM). The main endpoint is the adjustment of the disease -free survival (DFS), which is defined as the DFS that randomly 3 years. Secondary research finals are the general survival period (OS).

Figure 1. Data research design
Previous research results [5]
As early as October 11, 2017, Lancet Oncology has published Data research results online. From June 28, 2006 to August 10, 2009, 955 of the 1912 patients who were admitted to the group were distributed to the 3 -year group of Anenotzozole treatment, and 957 were allocated to the 6 -year group of Annotzole treatment. A total of 1860 patients were eligible (931 cases in 6 years and 929 cases in 3 years). At the median follow-up of 4.2 years, the 5-year adjustment DFS rate of the 6-year group was 83.1%, and the 3-year group was 79.4%(HR = 0.79, 95%CI 0.62-1.02, P = 0.066). In the first three years of random grouping, there was no difference in the incidence of adverse events in each group. In the entire observation period (0-6 years), compared with the 3 -year treatment group, all levels of joint pain or myalgia in the 6 -year treatment group (58% VS 3 -year group 53% in the 6 -year group) and osteoporosis ( The incidence of 21% vs 16%) is higher. Whether it is 3 or 6 years, there are no differences in cardiovascular incidents in each group. The adverse events observed are mainly level 1-2, and there are no cases of poisoning death.
ESMO update data interpretation in 2022
The ESMO conference announced the final analysis results. The updated research results show that the main study endpoint ADFS, Anusterazole treatment 6-year group compared with the 3-year group: the DFS rates adjusted in 10 years are 69.1%and 66%(HR = 0.86, 95%CI 0.72-1.01 , P = 0.073), ADFS's absolute benefit is 3.1%.
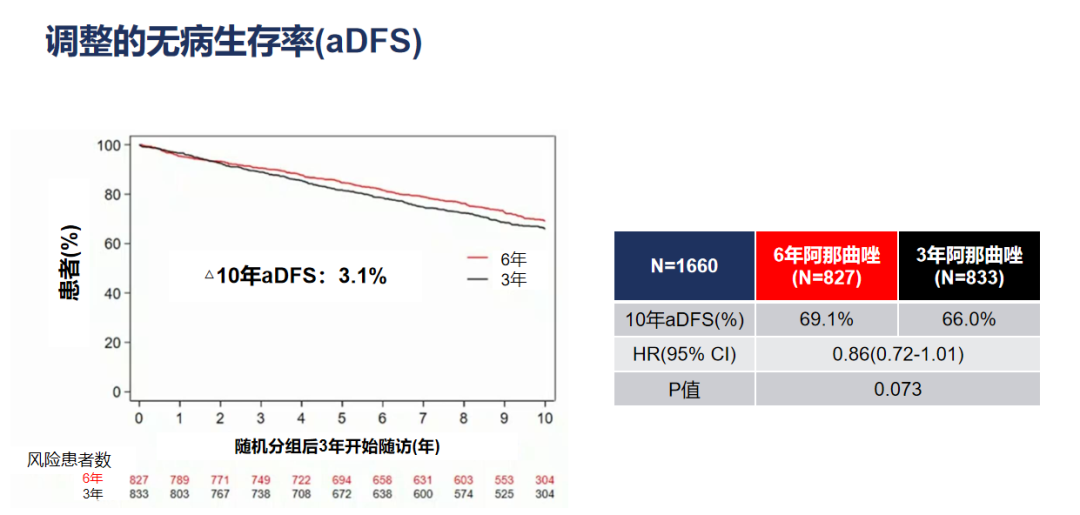
Figure 2. The adjustment of the adjusted DFS
Further sub -group analysis shows that the 6 -year group of Anenzozole treatment is compared with the 3 -year group:
Estrogen (ER) positive and progesterone (PR) positive: The DFS rate of 10-year adjustment is higher (70.8%vs 64.4%, HR = 0.77, 95%CI 0.63-0.94, P = 0.008);
ER positive or PR positive: The DFS rate of 10 years is similar (63.7%vs 70.9%, HR = 1.22, 95%CI 0.86-1.73, P = 0.28).
Compared with single -positive people, ER and PR dual -positive, the 10 -year adjustment of the 10 -year adjustment of the endocrine therapy group is significantly better (interactive p = 0.018).
In addition, for the positive lymph nodes and ER and PR two -positive patients (849 cases), compared with the 3 -year group in the 6 -year group of Annotazo, the DFS rate of 10 years is significantly higher (68.7% vs 60.7%, HR = 0.74 ,95%CI 0.59-0.93,P=0.011);对于淋巴结阳性、肿瘤≥2厘米且ER、PR双阳性患者(429例),阿那曲唑治疗6年组与3年组相比,10年调整The DFS rate of DFS is significantly higher (70.0%vs 56.4%, HR = 0.64, 95%CI 0.47-0.88, P = 0.005).


Figure 3. Figure 4. DFS benefits of patients with sub -groups
In terms of secondary research end, the 6-year group of Anusterazole was compared with the 3-year group: the OS rate of 10 years of OS was 80.9%and 79.2%(HR = 0.93, 95%CI 0.75-1.16, P = 0.53 ), OS's absolute benefit is 1.7%. Figure 5. The adjustment of the adjustment OS benefits
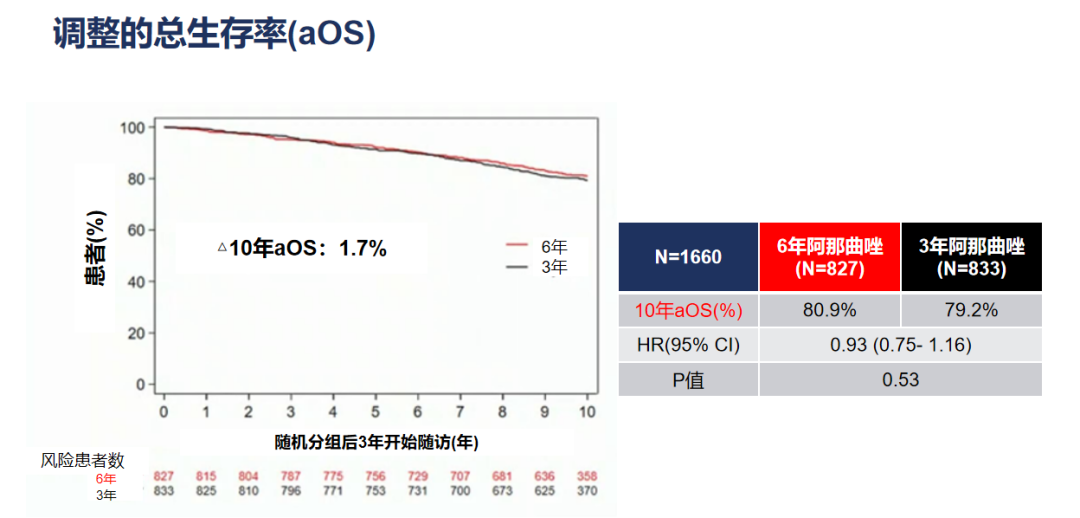
Further sub -group analysis shows that the 6 -year group of Anenzozole treatment is compared with the 3 -year group:
ER-positive and PR positive: The two-year OS rate for 10 years is 82.7%VS 78.7%(HR = 0.83, 95%CI 0.65-1.07).
ER or PR positive: The two-year-old OS rate is 75.2%vs 81.0%(HR = 1.33, 95%CI 0.86-2.05).
For patients with positive lymph nodes and ER and PR dual-positive patients, compared with the 3-year group in the 6-year group of Annotrazole, the OS rates adjusted in 10 years were 81.4%and 76.7%(HR = 0.84, 95%CI 0.62-0.93. P = 0.23); For patients with positive lymph nodes, tumors ≥2 cm, and ER, PR dual -positive patients, the 6 -year group of Anusterazole was compared with the 3 -year group. The 10 -year adjustment OS rate was 80.6%and 72.5%(HR = 0.71, 95%CI 0.48-1.05, P = 0.084).
Figure 6, Figure 7. OS benefits of patients with sub -group


Analysis conclusion
It is not recommended to use aromatase inhibitors for the use of aromatase inhibitors for more than 5 years for all postmenopausal patients with HR positive breast cancer. ER and PR dual -positive can effectively predict the efficacy of extended treatment and guide clinical decisions. At the same time, it can screen for people who can best benefit from extended endocrine therapy according to anatomical factors such as lymph nodes and tumor size.
Real world research reveals the decisive factors and effectiveness of extending assisted endocrine therapy
Study introduction
This study uses data from 6 health registers in Sweden. Based on patients, it aims to
Evaluate the occurrence of extension of assisted endocrine therapy and changes in its time;
Determine the clinical characteristics and patient factors that decide to use extended auxiliary endocrine therapy;
Explore the correlation between extending assistance endocrine therapy and survival ending in the real world.
Research forward-looking follow-up of 13168 patients with breast cancer (2005-2020), starting with their first prescription, he Moqifen or aromatic enzyme inhibitors, and classified them as extended or non-prolonged auxiliary endocrine therapy. COX regression analysis is used to evaluate whether the auxiliary endocrine therapy is related to the ending of breast cancer. The extension of the auxiliary endocrine therapy is defined as continuing to treat ≥6 months, and registered ≥ 2 times after 5 years of assisted endocrine therapy. The prescription information of his Moqifen or aromatic enzyme inhibitors comes from the Swedish prescription drug registration office.
Research result
1. The use of assisted endocrine therapy within 10 years
Of the 4,660 patients who completed 5 years of auxiliary endocrine therapy, 1386 patients (29.7%) patients extended the treatment. In line with this, within 6 months after completing the auxiliary endocrine therapy before the first 5 years, patients who continued to use auxiliary endocrine therapy dropped sharply from 57.7%to 20.0%.
2. Extend the occurrence of auxiliary endocrine therapy
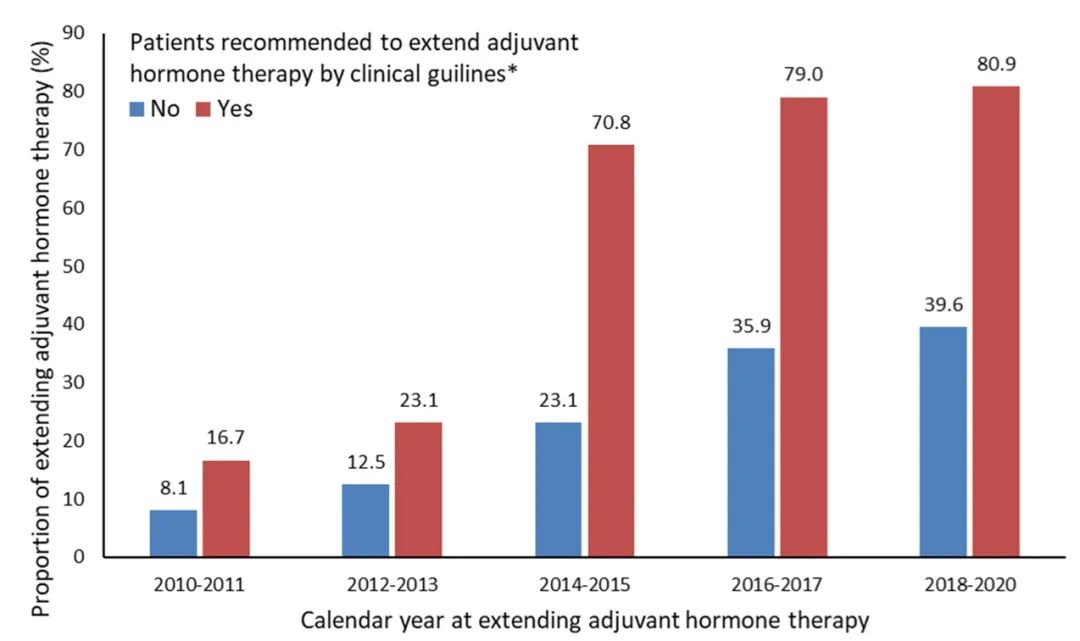
Over the past 10 years, the proportion of patients with extended treatment has increased sharply. Among the patients who meet the treatment standards for clinical guidelines, the corresponding proportion increased from 16.7%from 2010-2011 to 80.9%from 2018-2020. Among the patients who did not meet the extension treatment standards, the corresponding proportion also increased by nearly 5 times, from 8.1%in 2010-2011 to 39.6%from 2018-2020.
Figure 8. Whether the Swedish national clinical guide is recommended to extend the treatment according to 2010-2020, and determine the occurrence of extended treatment of patients who have completed 5 years of auxiliary endocrine therapy
3. Extend the prediction factors of assisted endocrine therapy
Diagnostic age, use of aromatic enzyme inhibitors (compared to him), large tumor volume, lymphaticity, high tumor grading, awareness of progesterone receptor state, HER2 state positive, chemotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radiotherapy, radilate The group of patients with first -level relatives died of breast cancer and high -income patients. In the age adjustment model, it is related to the higher possibility of extending the treatment after completing the 5 -year auxiliary endocrine therapy.
Among the multi -variable correction models, the age is young (<40 VS ≥65 years old), the lymph nodes are accumulated, the tumor grading is high (level 3 VS 1), chemotherapy, first -level relatives died in breast cancer, high income and income and income and income The correlation of extending assisted endocrine therapy is still statistically significant.
4. Extend the correlation between the auxiliary endocrine therapy and the ending of the survival
Among the patients who did not extend the auxiliary endocrine therapy, the incidence of DFS analysis was 26.8 (95%CI, 24.2-29.8)/1,000 people-20.6 of the patients who extended the treatment after 5 years of auxiliary endocrine therapy (95 (95 (95 %CI, 16.6-25.6)/1000 people-year. COX regression analysis shows that the time for assisted endocrine therapy is extended to DFS improvement (HR = 0.72, 95 CI% 0.55-0.95). However, when the prolonge and non-extended person who auxiliary endocrine therapy is compared, the statistical difference between OS is not found (HR = 0.94, 95 CI% 0.67-1.33). The sub -group analysis of tumor size, lymph nodes, tumor grading, or auxiliary endocrine therapy type shows consistent results at the baseline. Figure 9. Extend the DFS and OS of patients with auxiliary endocrine therapy and not extended auxiliary endocrine therapy
Research summary and discussion

The study found that in the past few decades, whether it meets or does not meet the extended standards of clinical guidelines, the proportion of women who extend the auxiliary endocrine therapy has increased by 5 times. Patients with poor tumor characteristics (positive lymph nodes, high tumor grading), patients with chemotherapy or first -level relatives who die from breast cancer are more likely to extend the auxiliary endocrine therapy for more than 5 years. The extended auxiliary endocrine therapy is related to better DFS benefits.
Since 2010, clinical guidelines have suggested that lymph node positive hemosfen users receive extended treatment [6-8]. In this study, nearly 90%of the lymph nodes in 2019 were positive for extended treatment, suggesting that the compliance of clinical guides was relatively good. Since 2018, clinical guidelines have suggested that aromatase inhibitor users extend the treatment [9,10]. However, in 2017, 71%of patients with lymph nodes positive diseases extended the auxiliary endocrine treatment time after 5 years of aromatase inhibitors. This shows that there is a lag between the existing evidence and the clinical guide, and the clinicians may have prescribed extended therapy for users of aromatase inhibitors based on their cognition. [11-14]
In the study, patients with chemotherapy who received chemotherapy received the possibility of extending assisted endocrine therapy for more than 5 times that of patients with chemotherapy. Accepting chemotherapy reflects poor tumor characteristics, such as positive lymph node, large tumor volume, high proliferation rate and/or HER2 state positive [15]. The result is consistent with the current clinical guide, that is, only the recommendation of patients with high recurrence risks to receive extended auxiliary endocrine therapy [9,10].
Patients with breast cancer who died in breast cancer extended 84%of the chance of assisting endocrine therapy for more than 5 years. It is expected that these patients are usually highly positive, so they may be more willing to extend the treatment time. The proportion of young women's extended auxiliary endocrine therapy is relatively high, which is related to the poor prognosis of these female patients [16,17]. Higher education and income can also increase the proportion of patients with extending assisted endocrine therapy. In general, the study shows that the severity and patient characteristics of the disease may affect the decision to extend the auxiliary endocrine therapy.
In addition, the DFS rate of patients with extended auxiliary endocrine therapy in the study has improved by 28%, which is equivalent to the effect observed in clinical trials. The study also shows that among the patients who have completed the treatment of aromatic enzyme inhibitors for 5 years, extending assisted endocrine therapy is related to DFS improvement. In short, the study explained from the perspective of real world research that extending the auxiliary endocrine therapy for more than 5 years may improve the end of breast cancer survival.
However, it should be pointed out that although extending assisted endocrine therapy is correlated with the risk of breast cancer incidents, it may also increase the risk of other diseases, such as cardiovascular disease and fracture [18]. Therefore, it may not be recommended that all patients with breast cancer receive extended auxiliary endocrine therapy.
Summarize
Data research and real -world research data jointly indicate that among patients who meet the clinical guidelines and do not meet the therapy standards of clinical guidelines, the proportion of patients with an auxiliary endocrine therapy has been extended to more than 5 years. Among them, DATA research has also determined that ER, PR dual -positive people, etc., extending assisted endocrine therapy can significantly improve benefits. Of course, the possible beneficiaries of patients who have extended the auxiliary endocrine therapy still need to be further verified.
Expert Introduction
Professor Liu Liping
Hunan Cancer Hospital Chief Physician

Members of Multi -disciplinary consultation group of breast cancer in Hunan Cancer Hospital
Deputy Chairman of the Hunan Health Management Society Breast Health Management Commission
Deputy Chairman of the Professional Committee of Maternal and Child Health and Equipment of Hunan Province
Deputy Chairman of the Hunan International Medical Exchange Promotion Association of the Breast Cancer Professional Committee
Standing Committee Member of the Hunan Provincial Association for Prevention and Medicine Prevention and Control
Standing Committee Member of the Breast Professional Committee of the Chinese Female Physician Association
Changjiang Academic Band Standing Committee Standing Committee
Member of the Gynecological and Breast Tumor Prevention Professional Committee of Maternal and Child Health and Equipment of Hunan Province
Member of the Hunan Anti -Cancer Association Molecular A targeted therapy
Members of the Expert Team of the Hunan Medical Society for the standardized diagnosis and treatment of breast cancer
references:
[1]. In 2022, CSCO breast cancer diagnosis and treatment guide
[2]. Zhou Yi, Liu Xiaoan. Early breast cancer extending the research progress of endocrine therapy [J]. China Oncology Surgery Magazine, 2022 (014-002). [3] .tjan-Heijenn VCG, Lammers SME, Geurts SME, et al Extended Adjuvant Aromatase inchibition after SequeentiaLeCrine therapy: Final Results of the Phase III DATA TRIAL. 2022 ESMO ABSTRACT 133O.
[4] .zeng e, he w, sjölander a, et al. Determinants and Effectiveness of Extending The Duration of Adjuvant Hormone Therapy Beyond 5 Years in Breast Cancer Res.
[5].Tjan-Heijnen VCG, van Hellemond IEG, Peer PGM, et al; Dutch Breast Cancer Research Group (BOOG) for the DATA Investigators. Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial . Lancet oncol. 2017 nov; 18 (11): 1502-1511.
[6] .Breast Cancer National Care Program. Regional Cancer Center; 2009.
[7].Aebi S, Davidson T, Gruber G, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology 2010;21 Suppl 5: V9-14.
[8].Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. Journal of clinical oncology : official journal of the American Society of clinical oncology 2010; 28: 3784-96.
[9].Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019; 37: 423-38.
[10] .Breast Cancer National Care Program. Regional Cancer Center; 2018.
[11] .goss people, Ingle Jn, PriceChard Ki, et al. EXTENDING AROMATASE-Inhibitor Adjuvant Therapy to 10 Years. The New ENGland Journal of Medicine. van Hellemond IEG, Peer Pgm, et al. Extended Adjuvant Aromatase inhibition after Sequential endocrine therapy (data): a randomised, phase 3 trial oncol 2017; 18: 1502-1111
[13].Mamounas EP, Bandos H, Lembersky BC, et al. Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): a randomised, double-blind, placebo-controlled, Phase 3 trial. The Lancet Oncology 2019; 20: 88-99.
[14].Del Mastro L, Mansutti M, Bisagni G, et al.Extended therapy with letrozole as adjuvant treatment of postmenopausal patients with early-stage breast cancer: a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2021 ; 22: 1458-67.
[15].Burstein HJ, Curigliano G, Loibl S, et al.Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Annals of Oncology 2019; 30: 1541-57.
[16].Bharat A, Aft RL, Gao F, Margenthaler JA. Patient and tumor characteristics associated with increased mortality in young women (< or =40 years) with breast cancer. Journal of surgical oncology 2009;100:248-51.
[17] .Azim Ha, Jr., PartRidge Ah. Biology of Breast Cancer in Young Women. Breast Cancer Res 2014; 16: 427.
[18].Goldvaser H, Barnes TA, Šeruga B, et al.Toxicity of Extended Adjuvant Therapy With Aromatase Inhibitors in Early Breast Cancer: A Systematic Review and Meta-analysis. JNCI: Journal of the National Cancer Institute 2017;110:31 -9.
Approval number: CN-103220 Expiration date:: 2023-9-23
Materials are provided by Astrikon, for reference for medical professionals


- END -
The local area is so big!"Limited" cool is about to go offline, and the temperature returns 30 ° C

Precipitation has not exited completelyThis evening to nightThere are still rainfa...
A pair of buses in Beijing was "shaved"
According to the Beijing Daily, on the morning of July 30, on the auxiliary road f...