What should I do with breast cancer dewracctic chemotherapy and endocrine therapy?3 major research branches!| 2022 ESMO
Author:Cancer Channel of the Medical Time:2022.09.22
*For medical professionals for reading reference

2022 ESMO cutting -edge progress
Hormonal receptor -positive (HR+) breast cancer (BC) accounts for about 70 % of all breast cancer. Endocrine therapy through the entire treatment cycle of HR+BC can be described as "gitzen" in reducing the risk of tumor recurrence. However, endocrine therapy regardless of the choice of drugs or the grasp of the treatment cycle. Today, which is increasingly emphasizing the accurate management of the disease today, it will undoubtedly become the focus of the attention of clinicians of breast cancer.
In 2022, the European Cancer Internal Science Association (ESMO conference) was successfully held in Paris from September 9th to September 13th. A number of heavy cutting -edge results about breast cancer were reported at the conference. These three important studies on endocrine therapy should not be missed ~
Sanyang BC backline de -chemotherapy plan new exploration!
Traditional division "spoiler" treatment effect!
IMonarcher II period: Abelley+Tushubu Mipido ± fluorivis group VS Qukoguzab+chemotherapy therapy HR+/HER2+ABC's final overall survival period (OS)
Summary number: LBA18
Monarcher is a phase II test with random, multi -center, open label, aiming to compare the selective selective CDK4/6 inhibitor Aboxili+Tushuzuke's anti -± fluorivis group and Tushubu monetary+standard chemotherapy Add the effect of adding to HR+/HER2+ABC. The conference announced the final OS results, as well as analyzing data from the exploration biomarkers in the inner subtype through RNAS sequencing data.
This study has received at least 2 types of anti -HER2 treatment in the past, hormone receptor positive HER2 positive breast cancer patients treated with CDK4/6 inhibitors and fluoros group, and random with 1: 1: 1 random It is divided into group A (Abercille+Telkuzumab+Fluvis group), Group B (Abbecilo+Telku Mipida) and Group C (Quzumab+chemotherapy). The main research final is no progressive survival (PFS), and the secondary research end point is an objective relief rate (ORR), safety, OS, patient report ending, and drug metabolism. The layered factors include whether the baseline lesions can be measured and the number of threads of the previous body. At the same time, analysis of the different internal subtypes of breast cancer through RNA sequencing data is analyzed for exploration of biomarkers.
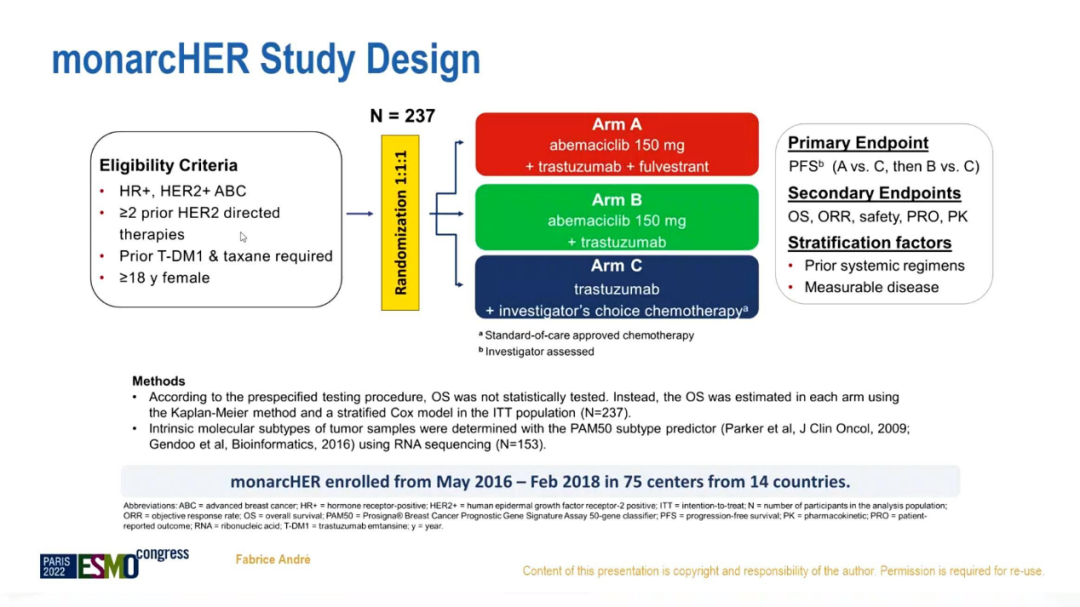
Research Process
The results showed that there were 157 deaths: 50 cases (63%) in group A, 54 cases (68%) in group B, and 53 cases (67%) in Group C. After the median follow-up time of 52.9 months, the MOS of the three groups were 30.3, 29.2, and 20.7 months (A vs C: HR = 0.71; 95%CI 0.48-1.05; bilateral P = 0.086; B VS C: HRR = 0.84; 95%CI 0.57-1.23; bilateral P = 0.365).
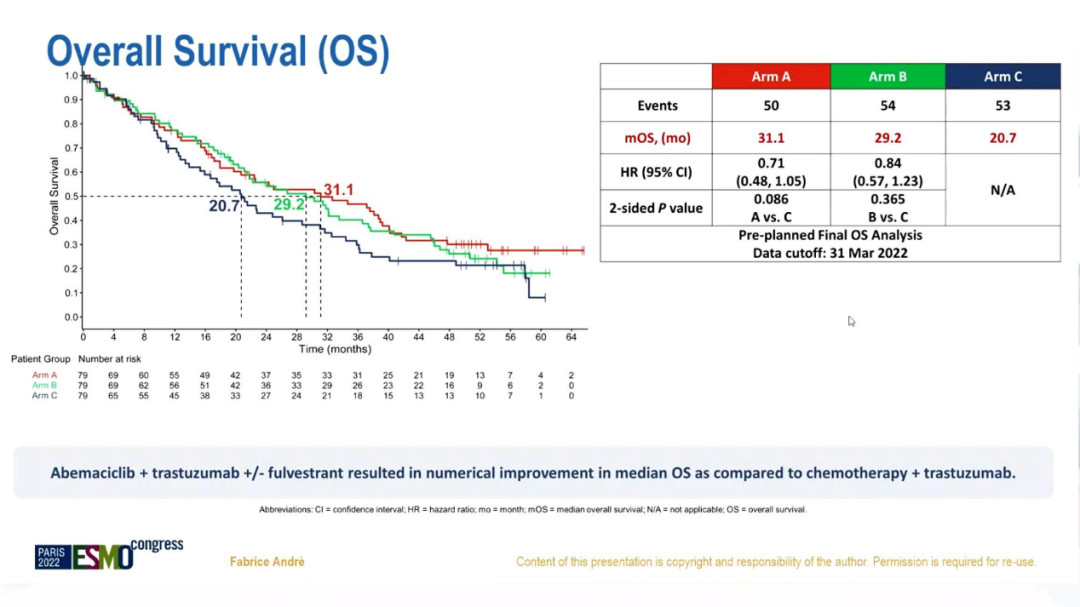
OS analysis
OS's sub -group analysis shows that compared with chemotherapy + Tushuzab, Abity + Flui Si group + Tushubu Mippitum is consistent in the preset sub -groups.
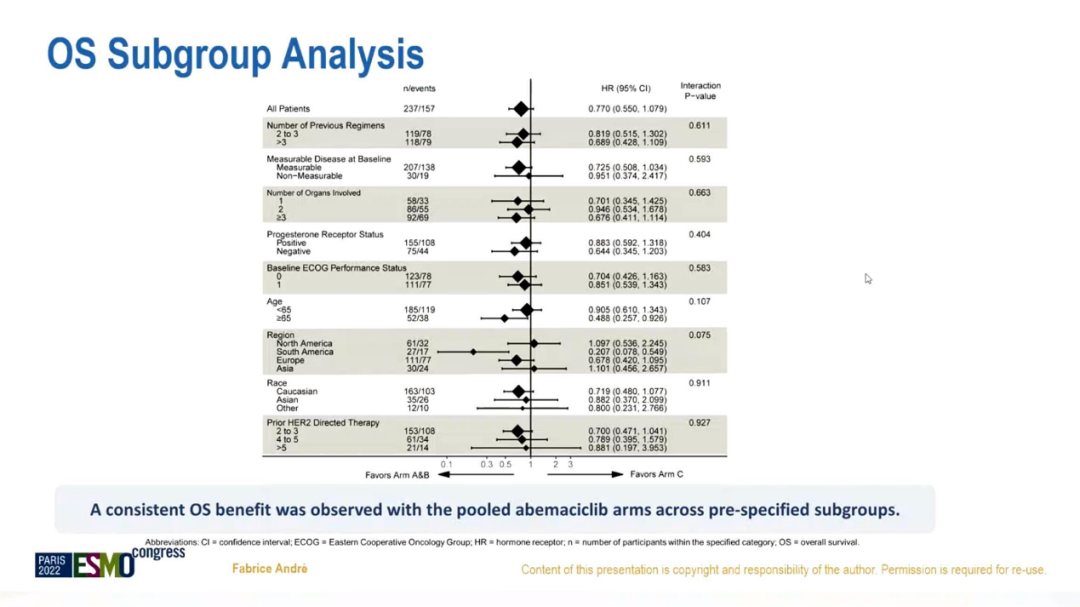
OS Asian group analysis
In the analysis of the exploratory RNA sequencing data, compared with the non-Luminal subtype, Luminal subtype PFS (8.6 vs 5.4 months; HR = 0.54; 95%CI 0.38-0.79) and OS (31.7 vs 19.7 months; HR = HR = 0.68; 95%CI 0.46-1) are long.
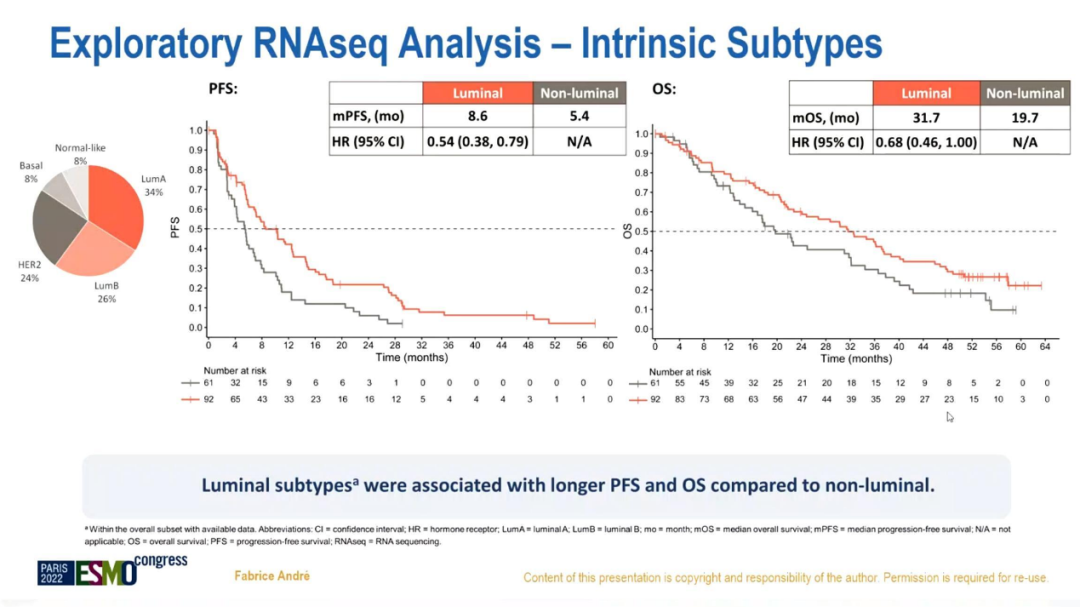
Explorest RNA sequencing data analysis
In terms of security, new security signals are not observed, which is consistent with preliminary analysis.
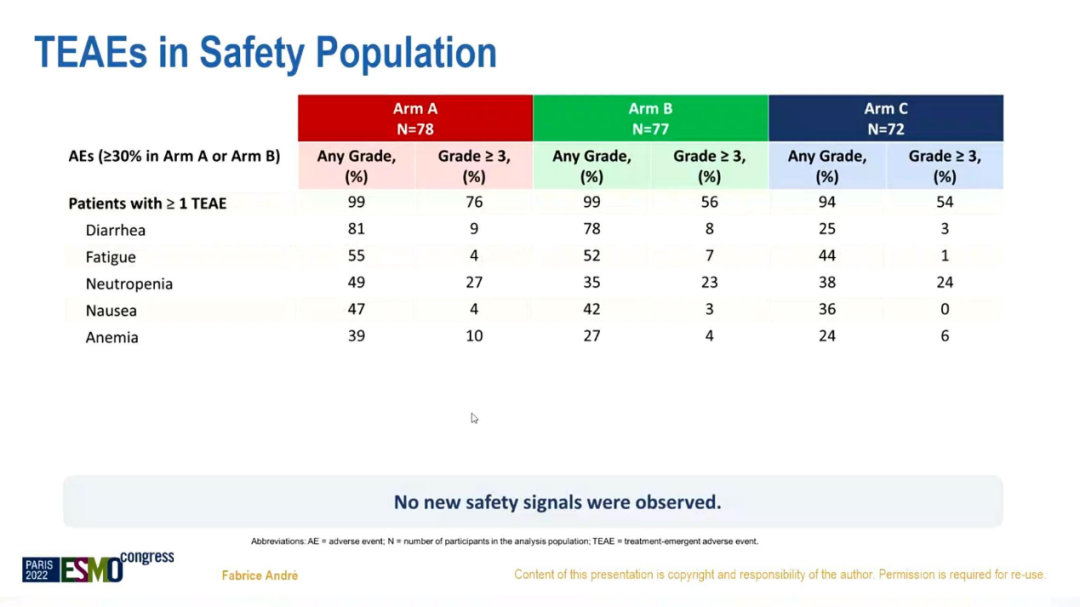
Adverse reaction data of the security group
In summary, compared with chemotherapy+Tushuzab, Abelley+Torcetab's ± Fluowis Group has improved the OS of HR+/HER2+ABC in terms of value. In the month, the risk of total death was reduced by about 16%-29%; safe and controllable, consistent with previous research; compared with non-Luminal subtypes, the Luminal subtype PFS and OS were significantly prolonged.
Early drug resistance or predictable?
New logo "Fingering" treatment plan!
Mutation feature analysis reveals the genome instability characteristics related to ET +/- CDK4/6I in ER+/HER2-MBC
Summary number: 210o
Apobec mutations are rich in MBC. However, among MBC patients, the prediction of APOBEC and other mutation processes has not yet been clarified. This study aims to explore genetic mutation features related to endocrine therapy (ET) +/-CDK4/6 inhibitors (CDK4/6i) in ER+/HER2-MBC.
The researchers retrieved the clinical and genome data of 4595 cases of breast cancer, and Panel, which passed the FDA certified Panel, for targeted sequencing. According to the explicit mutation process defined by Sigma, breast cancer is divided into Apobec+, HRD+or APOBEC-/HRD-typing. Use a single and multi -variable COX model to evaluate the PFS of any treatment line (L).
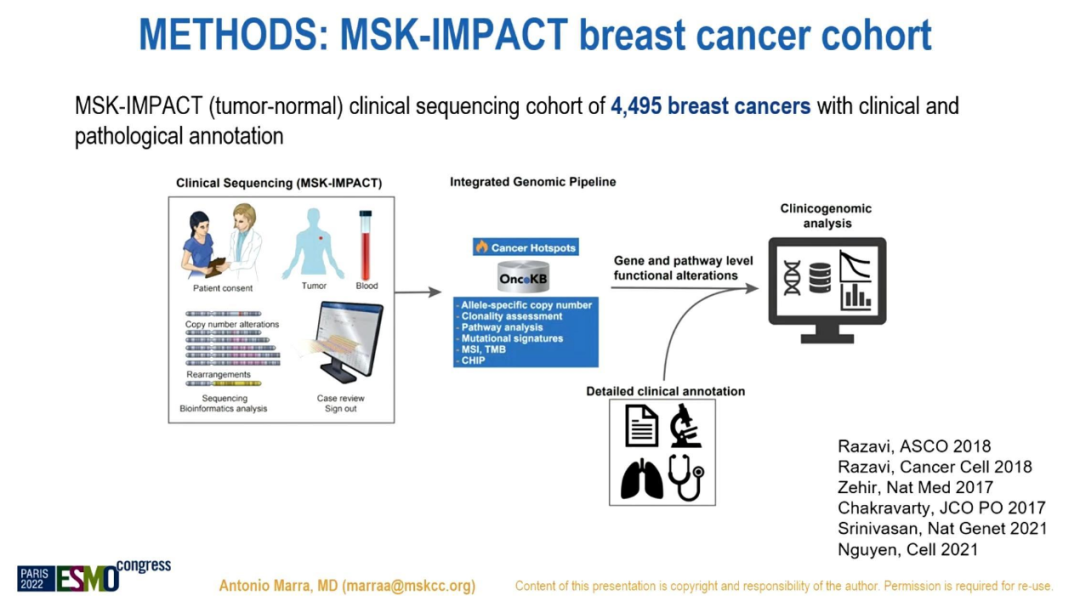
Research Process
The results showed that the proportion of APOBEC+and HRD+in MBC was higher than the primary tumors (24%and 24%vs 15%and 13%; P <0.001). APOBEC+is expressed in ER+/HER2- (19%) and HER2+subtypes (47%), while HRD+is expressed in three-negative subtype (36%). HR+MBC's APOBEC and HRD mutations feature
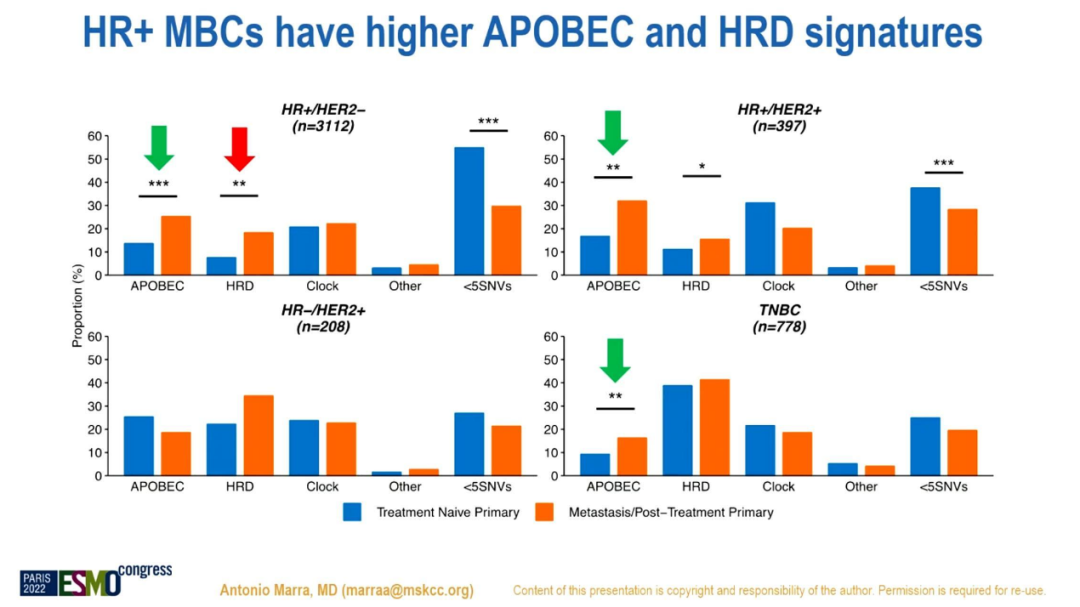
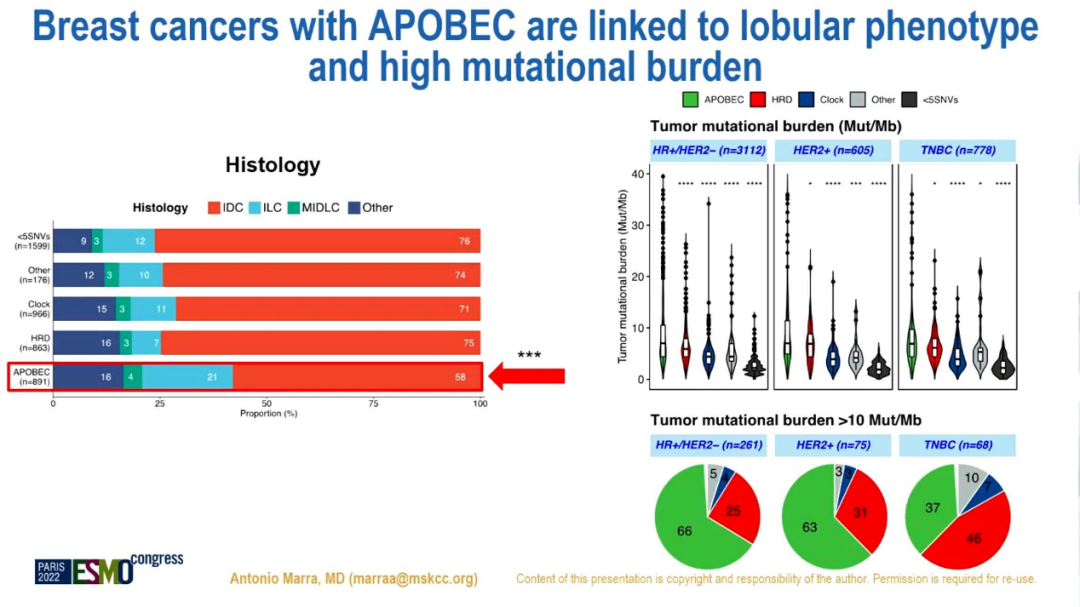
The ratio of APOBEC+in small leaflets is higher than the ratio of HRD+and Apobec-/HRD- (34%VS 10%VS 21%, P <0.001). ER+/HER2-MBC with APOBEC mutations shows lower ESR1 mutations (P <0.001).
APOBEC mutation is related to the lobular infiltration BC and high mutation load
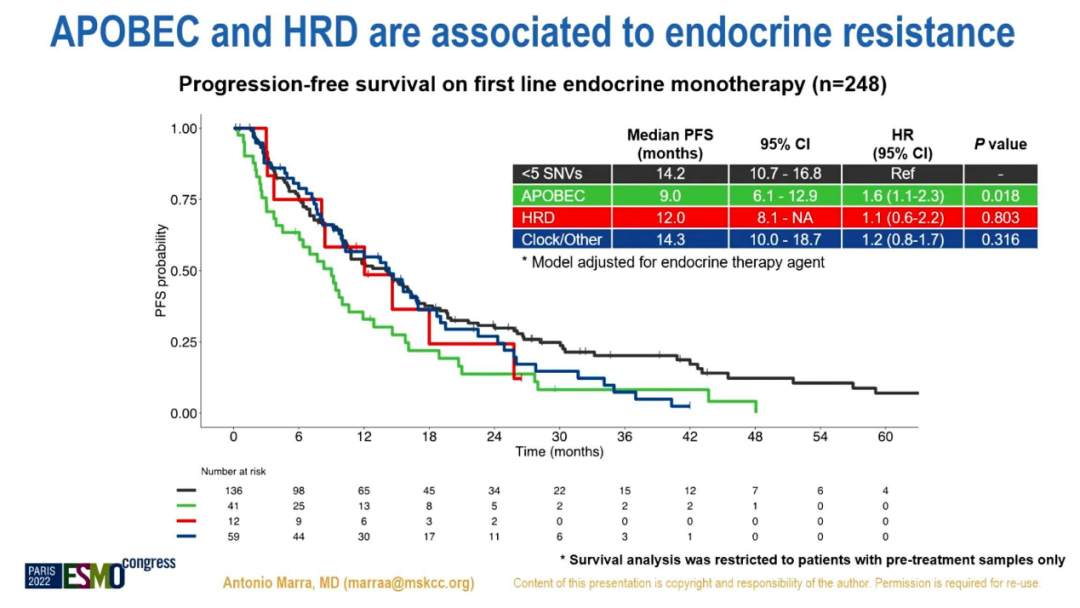
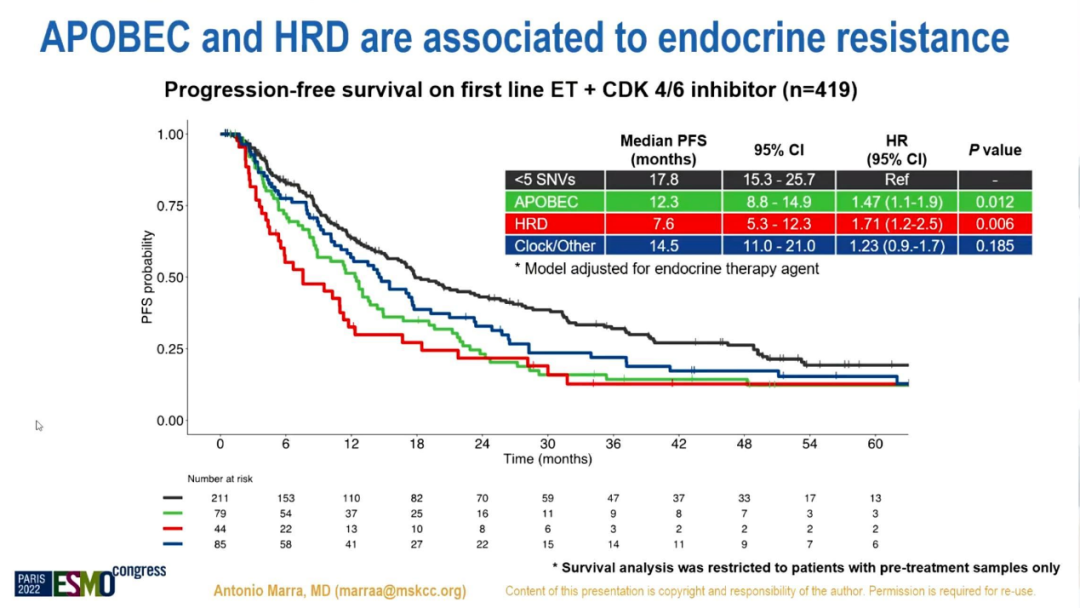
The survival analysis of patients with previous samples shows that in ER+/HER2-MBC (n = 395) that receives 1L or 2L ET single drug treatment, regardless of the type of ET drugs, Apobec+tumors are related to shorter MPFS independence (HR 1.6 , 95%CI 1.2-2.2, P = 0.001). In MBC (n = 419) treated with CDK4/6i+ET treatment in 1L, compobec-/HRD-, Apobec+and HRD+MPFS is lower (HR 1.5, 95%CI 1.1-2, P = 0.01 and HR 1.7, 95%CI 1.2-2.5, P = 0.006), and have nothing to do with the type of combined ET drugs. HRD+BC's MPFS treated with CDK4/6i+ET treatment at 2L (n = 207) and 3L (n = 372) decreases (HR 2.1, 95%CI 1-4.1, P = 0.03, HR 1.8, 95%CI 1.3 -2.5, P = 0.001).
The impact of mutation features on the treatment of first line ET single medicine treatment
The impact of mutation features on front line ET+CDK4/6i treatment PFS
In summary, patients with ER+/HER2-MBC of Apobec+and HRD+accept the short survival period of the ET +/- CDK4/6i treatment scheme. It is prompted that the genome instability brought by APOBEC and HRD can lead to early drug resistance and need to target these BCs. The sub -group tailor -made new therapy. At present, more genome analysis is being advanced to reveal mutation modes related to Apobec and HRD.
Is it necessary to extend endocrine therapy strategies?
These characteristics may be "filter" to benefit people!
治 Preface to endocrine therapy Extend the auxiliary treatment of aromathered enzyme suppression: the final result of the phase III DATA test
Summary number: 133O
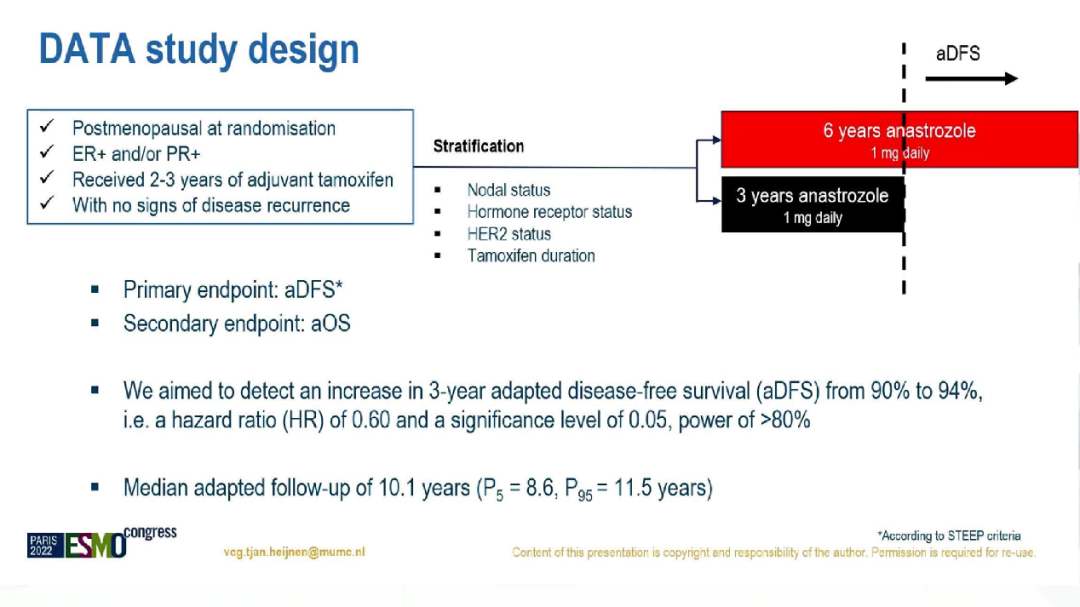
At present, for the postmenopausal HR+breast cancer, ASCO and ESMO guidelines are recommended for 5 years AI. He Moqifen sequels AI for 5 years, or 5 years in 5 years. However, whether the time to use AI for more than 5 years has brought clinical benefits without evidence. DATA research is a phase III, open label, and random control test, which aims to evaluate hormone receptor positive early breast cancer patients to use him for 2-3 years. The beneficiaries of Anxotzole treatment. The final analysis results were announced.
During the research period, a total of 1912 patients with HR+who had no recurrence or metastasis after the treatment of Moqifen's auxiliary treatment in 2-3 years will be randomly distributed to 3 or 6 years of the treatment group of Anenatozozozoi in a ratio of 1: 1 to 3 or 6 years. Essence Layer according to lymph nodes, hormone receptor status, HER2 status, and the duration of the past treatment of Mosfen. The main ending is the survival of no disease (ADFS) after correction, which is defined as DFS after 3 years of randomization. The secondary end is the general survival period after correction (AOS).
Research Process
In the end, a total of 1,660 patients met the conditions for 3 years after random grouping and had no recurrence. Among them, lymph node positive patients account for 2/3, and ER and PR dual -positive patients account for 3/4.
Patients and tumor characteristics
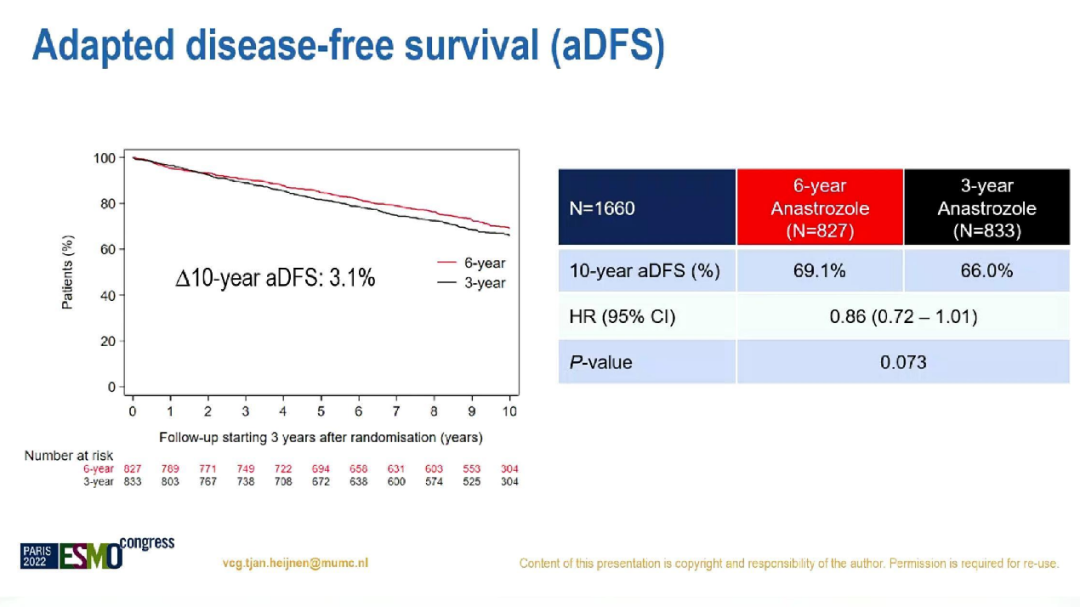
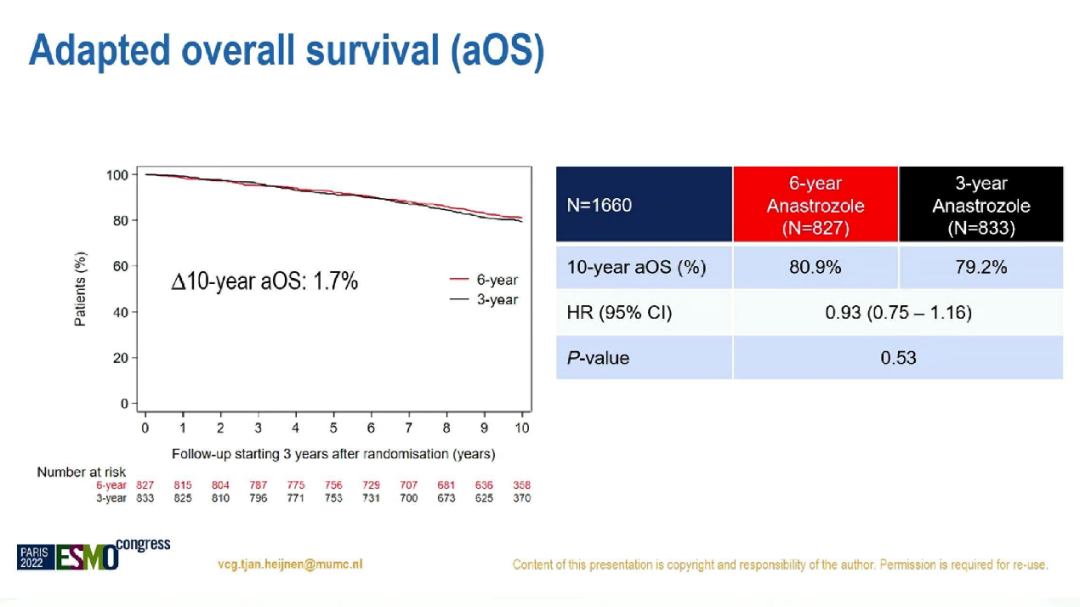
After the median correction, the 10.1-year follow-up, the 10-year ADFS of the 6-year and 3-year group was 69.1%and 66.0%(HR = 0.86; 95%CI: 0.72-1.01; P = 0.073), 10 years AOS AOS There is no significant difference, which is 80.9%to 79.2%(HR = 0.93; 95%CI: 0.75-1.16; P = 0.53).
Two groups of 10 years ADFS
10 -year AOS in the two groups
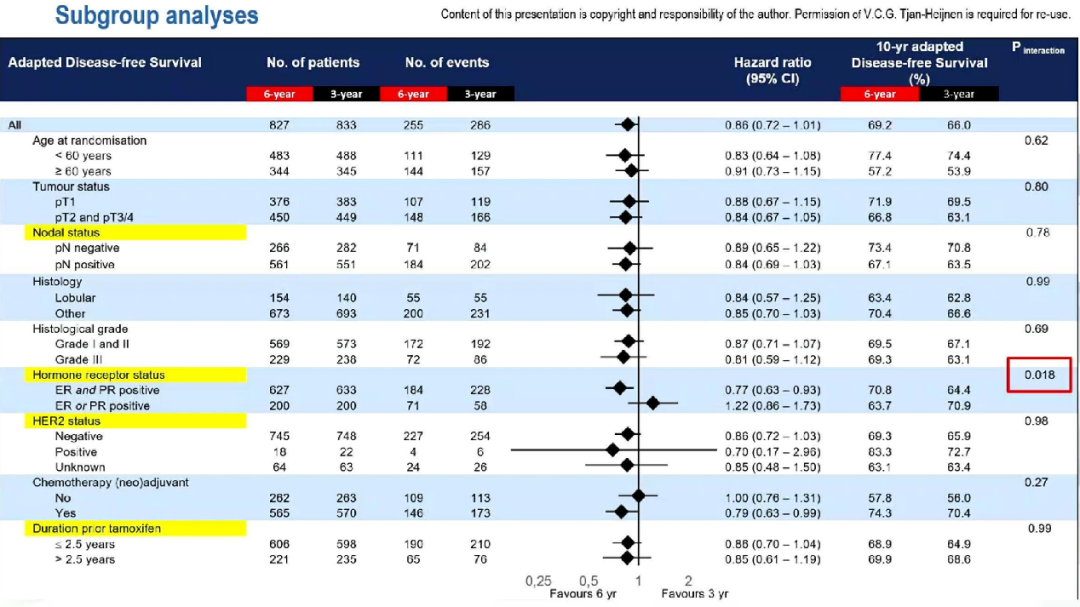
The layered analysis shows that among the ER and PR dual-positive tumors, the 10-year ADFS of the 6-year group is significantly higher than the 3-year group (HR = 0.77; 95%CI: 0.63-0.94; P = 0.008); Among the positive tumor patients, 10 years of ADFS in the two groups (HR = 1.22; 95%CI: 0.86-1.73; P = 0.28). Compared with the single -positive group of hormone receptors, the 10 -year ADFS benefit of the extended treatment is significant (interaction P = 0.018).
ADFS's sub -group analysis data
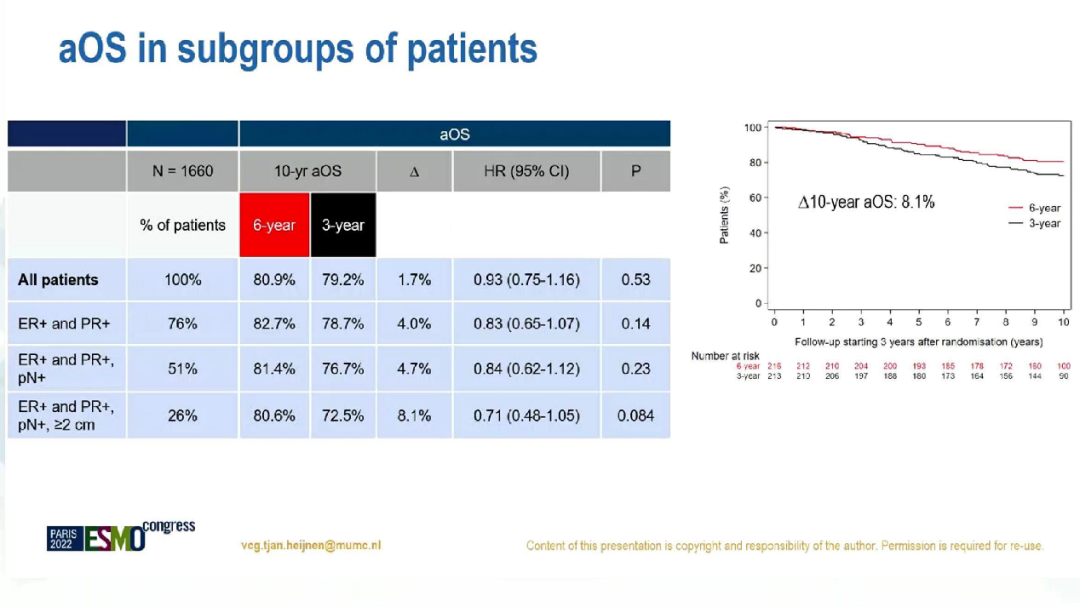
In addition, among patients with positive lymph nodes and hormone receptors (N = 849), 10 years of ADFS of 6 years and 3-year groups were 68.7%and 60.7%(HR = 0.74; 95%CI: 0.59-0.93; P = 0.011), there is no significant difference in AOS (HR = 0.84, 95%CI: 0.62-1.12; P = 0.23). ADFS's sub -group analysis
AOS's sub -group analysis
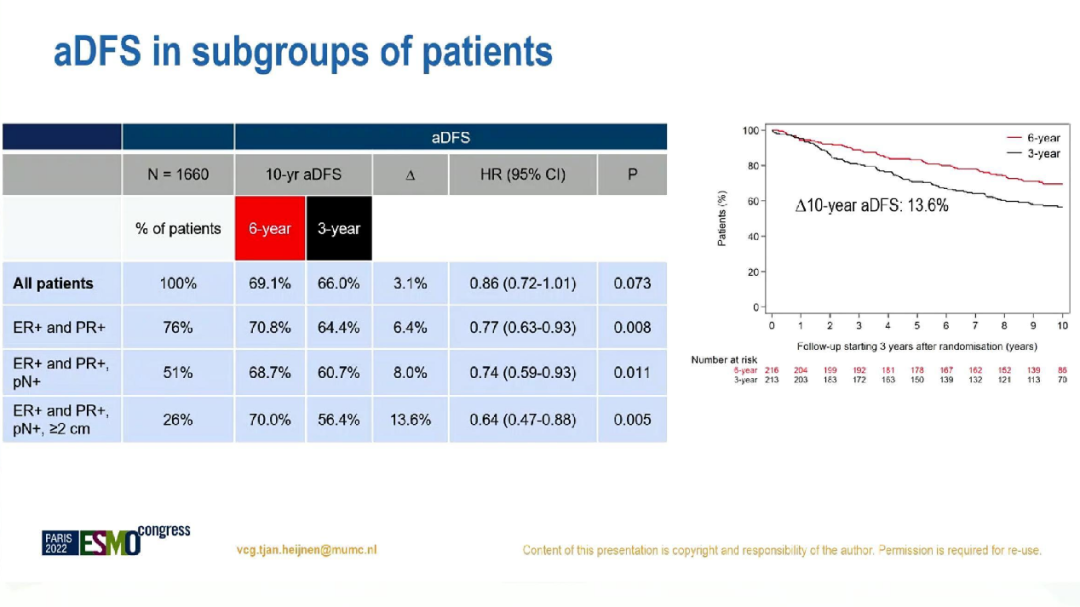
In summary, it is not recommended to extend the sequential treatment of aromatic enzyme suppression among all postmenopausal patients with HR+for more than 5 years. However, lymph nodes are positive, ER and PR bilateral tumor patients may benefit from extending treatment.
With the continuous update and iteration of endocrine therapy drugs, the 5-year survival rate of Chinese breast cancer patients has steadily increased from 73.1%between 2003-2005 to 82.0%from 2012-2015. It has also reached 90%. However, the pace of researchers to capture HR+breast cancer never stops: for example, drug development from a classic selective ER registered agent, hemosfen, aromatic enzyme inhibitors to sonazo, Anenotazo, to new selective selectivity ER receptor downside fluoros, Ellazqun, etc.; The three major studies above cover the prolonged treatment of early breast cancer, the selection of primary and second lines of advanced breast cancer and the selection of drug resistance and crowd screening.
At present, there are still many studies in the field of breast cancer endocrine therapy. It is expected that they can bring strong treatment effects, better quality of life, and more accurate design of endocrine therapy drugs and solutions in the future.
The first release of this article: the medical world tumor channel
Author of this article: lily
Editor in charge: Sweet
- END -
salute!salute!salute!

Today 95 years ago todayNanchang StreetA gunshot cuts through the silent night sky...
"Party Member" Three Light and Three Comparison "in Yutan Street: More than 1,500" silver age "brave as a pioneer

In July of this year, Yutan Street sounded the Sanliang Three Comparison operation...