K-medicine, a "long survival" PD-1 inhibitor
Author:Cancer Channel of the Medical Time:2022.09.22
*For medical professionals for reading reference

ESMO announced the long -term OS follow -up data of Paborzab's treatment of "pan tumor".
The annual meeting of the 2022 European Cancer Internal Science (ESMO) Annual Meeting, which was closed on September 13, announced a number of PD-1 immune checkpoints inhibitors Paborzab (commonly known as "K-drug" in China) for long-term survival (commonly known as "K drug") to treat different cancers (commonly known as "K drugs"). OS) follow -up data, including:
K-drug combined with platinum-containing chemotherapy first-line therapy to metastatic non-scale and squamous non-small cell lung cancer (NSCLC) Global Multi-Center III Phase III Clinical Research Keynote-189 and Keynote-407's five-year OS follow-up data [1,2]
K-medicine single-line treatment and combined chemotherapy first-line treatment of recurrence or metastatic head and neck scalescotenomic (HNSCC) phase III clinical research Keynote-048 [3]
The KEYNOTE-240 of the second-line therapy of the K-drug single-medicine treatment of non-removed liver cell carcinoma (HCC) is followed by OS [4], and K-drug second-line/back-line treatment of HNSCC Keynote-040 6 years of OS follow-up data [5].
These five keynote are multi -centered and randomly controlled phase III clinical research, but the indications they brought by them have been approved very differently. Sighing sighs. However, the exploration of long -living survival in the Keynote research series allows them to return to the same way, and jointly show the mystery of the survival of immunotherapy.
"Priority choice"
The first Keynote-189 and Keynote-407, which first announced the results of the research results in 2018, are the first global centers of the advanced NSCLC in the advanced NSCLC in the immunomotive checkpoint inhibitors to reach the main research end. During these four years, the research results rewritten the treatment guidelines for advanced scales and non -scale NSCLC including China and the United States, replacing platinum -containing chemotherapy, and became the standard front -line treatment of such patients.
It is worth mentioning that in the latest version of the US NCCN NSCLC Treatment Guide (2022 V4) released on September 2 this year, K drug combined with platinum chemotherapy is still the only treatment PS score specified by Preferred 0- 1 late transfer scales or non -scale NSCLC solutions. Although there are many other combined treatment solutions, although there are also large -scale III clinical research results "endorsement", they only have the designated "other recommend".
Why is the NCCN guide so "love" Keynote-189 and Keynote-407?
The results of repetitive research produced by high -quality clinical research institutes in the field of advanced NSCLC treatment (including long -term OS follow -up research results), relying on high -level evidence -based medical evidence evidence that should be the most important reason Essence
Taking Keynote-189 as an example, the complete statistical analysis plan requires two mid-term analysis and one final analysis. The three analysis results have verified the stability of the research data at the dual main endpoints of OS and PFS, and the analysis results of the secondary endpoint index. The long -term OS follow -up data analysis announced by ESMO proves for the first time that the immunomotive combined platinum chemotherapy scheme can bring better 3 years (31.3% vs 17.4%) and 5 years (19.4% vs 11.3%) than the pricing chemotherapy scheme. OS benefits.
K-drug is currently the only PD-(L) 1 inhibitor that announced its combined with platinum-containing chemotherapy first-line NSCLC 5-year survival data. There is no doubt that the long survival data of Keynote-189 and Keynote-407 will be "reached" by the 2022 V5 version of the NCCN NSCLC guide, together with the five-year OS data of Keynote-001, Keynote-024, and Keynote-010, continue Lock K medicine as "first recommendation".
Winning gene+brave heart =
Keynote-048
Like Keynote-189 and Keynote-407, Keynote-048 also evaluates the efficacy and safety of the first-line treatment of K-drug combined with platinum chemotherapy (cisplatin+5-FU) scheme, but the "main battlefield" is recurrence or metastasis header Cervical squamous carcinoma (R/M HNSCC). Another unique thing is that Keynote-048 also compares K-drug single drugs and anti-EGFR targeted drugs Sitenab combined with the Extreme scheme [6,7]. Challenging the PD-1 single medicine to challenge the targeted and platinum chemotherapy combined scheme is a bold and unexpected attempt.
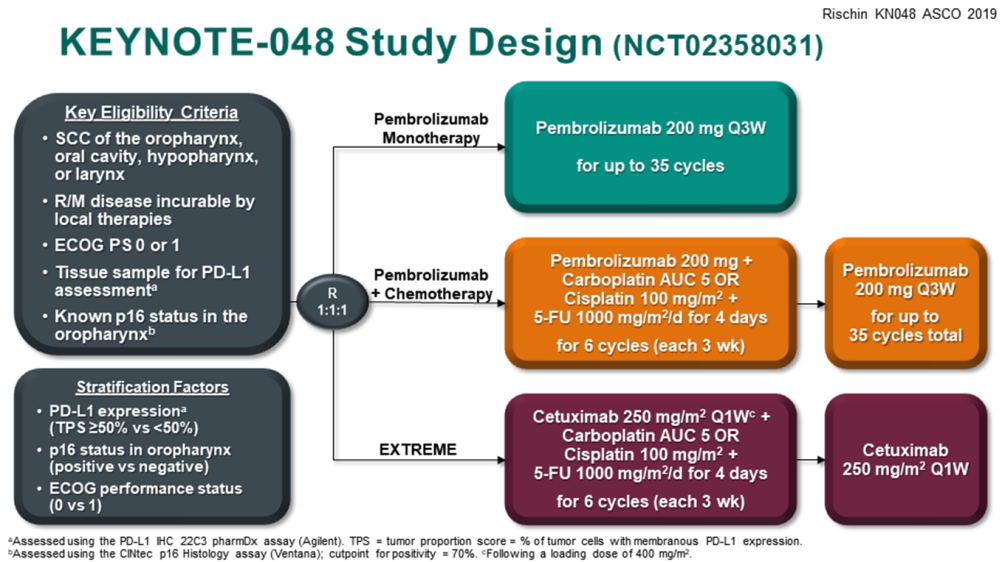
Figure 1 Keynote-048 Research Design
This study is aimed at PD-L1 CPS ≥ 20, CPS ≥ 1, and all patients. The three groups of groups of patients use total survival (OS) and non-progressive survival (PFS) as the common endpoint, and a total of 14 are set up in "one breath". The main research assumptions of statistical analysis are arranged.
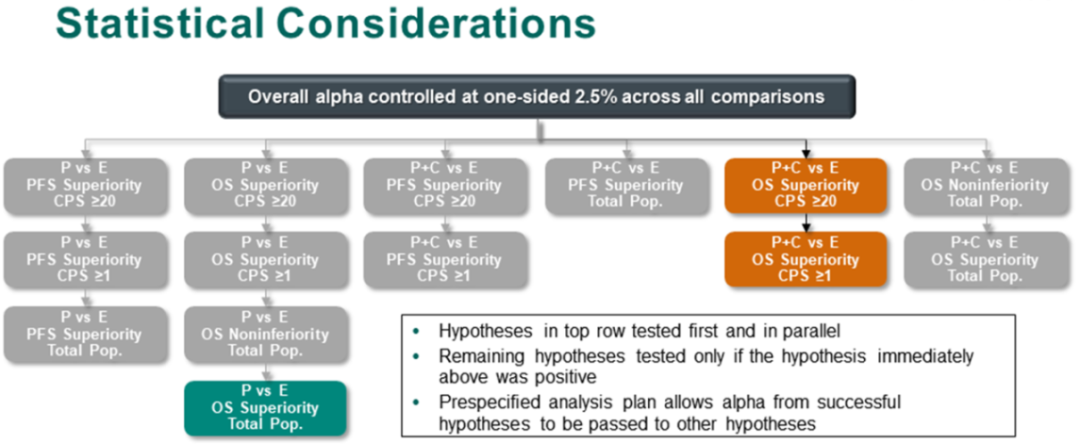
Figure 2 Keynote-048 Research Asian Group
The study announced the results of the mid -term analysis at the ESMO conference in 2018, and in 2019 ASCO announced the final analysis results. The results show that in the general population and PD-L1 CPS ≥ 20, CPS ≥ 1 population, K drug combined with chemotherapy "crushing" Extreme scheme can bring significant OS improvement and decrease in death risk. Moreover OS in PD-L1 CPS ≥ 20 and CPS ≥ 1 also better than targeted combined chemotherapy solutions, showing non-inferiority in the general population. The OS data results from the Keynote-048 follow-up of the Keynote-048 followed by the ESMO-048 followed by 69.2 (61.2-81.6) a month show that the first-line therapy of the K-drug single-line treatment PD-L1 CPS ≥ 20 people, PD-L1 CPS ≥ 1 population, and the overall population 5 years The OS rate was 19.9%, 15.4%, and 14.4%, respectively, superior to 7.4%, 5.5%and 6.5%of targeted combined chemotherapy schemes [3].
The 5 -year OS rate of the above -mentioned different people in the first -line treatment of K -drug combined with chemotherapy also reached 23.9%(6.4%in the control group), 18.2%(4.3%in the control group) and 16.0%(5.2%in the control group) [3]. In terms of safety, the adverse reactions related to the 3-5 treatment of K-drug single drugs and K drug combined chemotherapy groups were 17.0%and 71.7%, respectively, and the Extreme scheme was 69.3%[3].
The five-year OS follow-up data result of Keynote-048 once again verified the superiority of the K-drug single drug or the K-drug combined chemotherapy first-line treatment R/M HNSCC compared to the Extreme solution, and supported it to become a standard treatment scheme for the first-line treatment R/M HNSCC. At the same time, Keynote-048 According to the analysis of PD-L1 CPS layered results, there is a certain relationship with PD-L1 expression with PD-L1 according to PD-L1 CPS. Benefit.
"Long Survival" PD-1
The K-drug indications applied for KEYNOTE-189, Keynote-407 and Keynote-048 have been approved in China, the United States and the European Union. If the 5-year OS follow-up data of these studies allows doctors to answer the probability of using PD-1 survival in clinical practice, then the Keynote-240 released by ESMO this time, and second-line treatment of R/M HNSCC phase III clinical research The long-term OS follow-up data of Keynote-040 cannot actually determine the important value behind its research from whether the OS benefits are statistically significant.
Keynote-240 is a global III clinical study that cannot be removed from the second-line treatment of K-drug single medicine. The results announced by ASCO in 2019 show that compared with the best support treatment of the control, OS and PFS show the improvement of clinical significance, but it has not reached a statistically significant P value.
Similarly, Keynote-040 is a phase III clinical study that compares K drug and standard therapy for the second-tier treatment plan. Although OS also shows the improvement of clinical significance, it is also due to the failure to reach the preset statistical indicators. "Failure" ended.
Although it failed to bring the approval of indications, the exploration of Keynote-240 and Keynote-040's exploration of the long-term survival significance of K drugs seemed to give these two new life and significance.
In the KEYNOTE-240, the data results of 53.9 (46.1-63.1) of the K-drug single drug group showed that its 3 and 4 years of OS rates were 17.9%and 11.1%, respectively, which was better than the control group [medium position. 13%and 6.6%of the follow-up of 54.1 (46.0-62.2) months] [4].
The follow-up results of Keynote-040 showed that the median follow-up 74.8 (68.8-85.4) months, K-drug second-line treatment R/M HNSCC ITT crowd, PD-L1 TPS ≥50%, and PD-L1 CPS ≥1 groups The 6 -year OS rate is 6.5%, 8.9%, 7.1%, and is better than 2.4%, 6.3%and 2.1%[5].
Keynote's long-term exploration of "failure" research is not limited to Keynote-240 and Keynote-040. Exploring the survival of 3 years, 5 years, or more, has become the "standard" of all III research on all III. Regardless of the results of the research, whether the indications are approved or not.
K-drug has been approved in China to obtain 8 treatment indications, covering NSCLC, HNSCC, melanoma, esophageal cancer, and colorectal cancer, and Keynote, which supports these indications approved, has been marked with "long survival" [1- 3,8-11].
Figure 3 Research Summary of some series of keynote
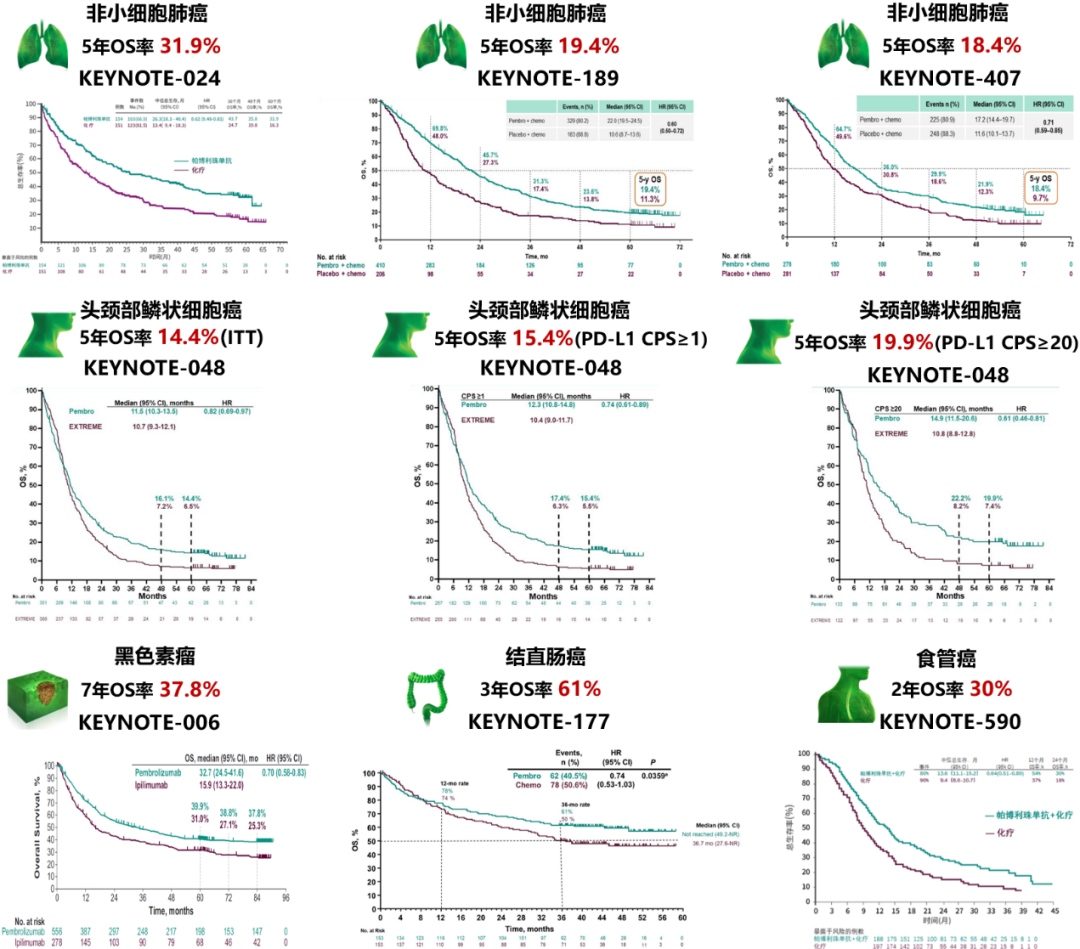
Among them, Keynote-006 is currently published the longest OS follow-up data. The results of the OS follow-up data of the 85.3 months of the KEYNOTE-006 studies released in 2021 also showed that the 7-year OS rate of patients with melanoma patients with a 7-year OS rate of patients with melanoma was as high as 37.8% (the control group was 25.3%). There is a "tail effect" of immunotherapy. The longer the tracking time, the more prominent the survival advantage is compared to the control group. In this case, the median OS cannot accurately and comprehensively reflect the efficacy of immunotherapy and the survival value it brings. Therefore, the survival rate of 3, 5 years, or 10 years is undoubtedly the hardest indicator of the value of immunotherapy brought about survival value.
At present, 14 PD- (L) 1 inhibitors have been approved in China for a total of 55 treatment indications (including MSI-H/DMMR solid tumor-limiting indications), covering 12 different physical tumors and hematological tumors Essence
PD- (L) 1 inhibitor will still expand rapidly, penetrate more tumor species, and absorb more combined treatment plans; but while the "universe" expansion is expanded horizontally, immunotherapy also needs long-term OS follow-up research like Keynote, and explores immunity in depth. Treatment of long survival to patients, so that more PD-(L) 1 inhibitors can be PK with K-drugs, and compete for "long survival" PD-1 "laurel".
Reference materials:
[1].M.C. Garassino et al., KEYNOTE-189 5-year update: First-line pembrolizumab (pembro) + pemetrexed (pem) and platinum vs placebo (pbo) + pem and platinum for metastatic nonsquamous NSCLC, 2022 ESMO, Abs #973mo
[2] .s. Novello et al., 5-year update from keynote-407: PEMBROLIZUMAB Plus Chemotherapy in Squamous Non-Small Cell LUNG CANCER (NSCLC), 2022 ESMO, ABS # 974Mo
[3].M. Tahara et al., Pembrolizumab with or without chemotherapy for first-line treatment of recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): 5-year results from KEYNOTE-048 2022 ESMO , ABS #659MO
[4].J. Edeline et al., Pembrolizumab (Pembro) vs placebo (Pbo) as second-line treatment for sorafenib-treated advanced hepatocellular carcinoma (aHCC): 4.5-year follow-up from KEYNOTE-240 2022 ESMO, Abs # 713p
[5].D. Soulieres et al., Pembrolizumab (pembro) vs standard-of-care (SOC) in previously treated recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): 6-year follow- Up of Keynote-040 2022 ESMO, ABS # 658MO
[6].Burtness B, Harrington KJ, Greil R,et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019 Oct 31. pii: S0140-6736(19)32591-7.[7].Rischin et al., Protocol-Specified Final Results of the KEYNOTE-048 Trial of Pembrolizumab as First-Line Therapy for Recurrent/metastatic head and next note
[8].Martin Reck et al., Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50 J Clin Oncol. 2021 Jul 20;39(21):2339 -2349. Doi: 10.1200/JCO.21.00174. EPUB 2021 APR 19
[9].C. Robert et al., 7-Year Follow-Up of KEYNOTE-006: Pembrolizumab Versus Ipilimumab in Advanced Melanoma, Presented at the 18th International Congress of the Society for Melanoma Research; October 28-31, 2021.
[10].Andre T et al., Final overall survival for the phase III KN177 study: Pembrolizumab versus chemotherapy in microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC). 2021 ASCO
[11].Metges JP et al., FIrst-line Pembrolizumab Plus Chemotherapy Versus Chemotherapy in Advanced Esophageal Cancer: Longer-term Efficacy, Safety, and Quality-of-life Results From the Phase 3 KEYNOTE-590 Study, 2022 ASCO-GI ORAL Presentation
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
Study and implement the spirit of the Provincial Party Congress Jining City Administration to improve the management level
Poster 丨 Niged "9 · 5" Luding 6.8 earthquake rescue power

After the 9 · 5 Luding earthquake occurredThe rescue force in all directionsSmell...