2022 ESMO | ADaura Research Data Update: Early EGFR mutations positive NSCLC patients DFS continued to benefit significantly!
Author:Medical community Time:2022.09.15

Summary
A few days ago, in 2022, the European Cancer Internal Science Association (ESMO) Annual Conference was officially held in Paris, France. Under the high expectations of the colleagues of many oncology community, there are many heavy studies in the field of lung cancer, of which the update of Adaura's research has attracted much attention. In the past, ADAURA research appeared in 2020 with an overwhelming effect. [1] pushed the auxiliary targeted therapy to a new peak. In the next 2 years, Adaura studied the age of the era and continued to refresh people’s people’s’s’s. expect. The latest data of the ADaura Study at the ESMO Conference has officially announced the latest data for nearly 4 years [2], which once again confirmed the extraordinary position of Oshitinib in the field of NSCLC auxiliary targeted therapy in the epidermal growth factor receptor (EGFR) mutation. In order to further understand the research data and broaden the clinical diagnosis and treatment ideas, on this opportunity, Professor Wu Yilong was invited to conduct in -depth interpretation to readers readers.
Adaura research constantly plowing new
ADAURA research is a global phase III dual-blind random control clinical study, which aims to explore Oshitinib as auxiliary therapy for the IB-IIIA phase EGFR mutation (19DEL/L858R), complete resection (R0 resection) The efficacy and safety of patients with NSCLC. The main data of this study --- No In progress (DFS) was the first to be announced at the 2020 American Clinical Oncology Society (ASCO) Annual Conference [1], and published the full text online (MDFS Oshitinib "on the" New England Medical Magazine "in the same year The group has not reached; the 2-year DFS rate: II-IIIA Phase 90%VS. The placebo group 44%, HR = 0.17, P <0.001; IB-IIIA Phase 89%vs vs. Comfort comfort 52%, HR = 0.20, P <0.001) [3]. Based on this, Oshitinib has been approved by the US FDA and the National Drug Administration (NMPA) for postoperative assistance for EGFR mutations. NSCLC auxiliary targeted therapy drugs. As a result, Oshitinib opened a new era of EGFR mutation positive NSCLC targeted auxiliary therapy.
Since then, Adaura has announced more data, and has demonstrated many advantages of Oshitininib auxiliary treatment plan from different dimensions: the results of the ADaura exploration analysis released by the 2020 World Lung Cancer Conference (WCLC) showed that in the overall crowd, regardless of the previous crowd, regardless of the previous crowd, regardless of the previous crowd, regardless of the previous crowd, regardless of the previous crowd, regardless of the previous crowd, regardless of the previous crowd, regardless of the previous crowd, regardless of the previous crowd, regardless of the past, regardless of the past, regardless of the previous crowd Whether you have undergone auxiliary chemotherapy, all Osicininib auxiliary therapy has significant DFS benefits (II-IIIA Phase DFS HR: Past Chemotherapy C first 0.14 VS. Non-chemotherapy 0.15) [6]; The patient's self -assessment of quality of life was analyzed, and the results showed that during the treatment of Oshitininib, the patient's health -related quality remains unchanged while obtaining a significant effect. During the ESMO meeting in the same year The results of the Asian group analysis, suggesting that Oshitinib assist therapy can significantly increase the CNS DFS and reduce the risk of CNS recurrence or death of 82%[8]; in this year's European Lung Cancer Conference (ELCC), ADAURA research announced China Asia The analysis of data, the results show that the efficacy and safety data of the Chinese Asian group (IB-IIIA) are consistent with the global population. Osicinib auxiliary treatment significantly reduces the risk of recurrence or death of patients by patient Clinical benefits [9],
Judging from the data disclosed at each stage, Oshitinib assisted the overwhelming DFS benefits and reduced the risk of CNS recurrence. It shows a significant curative effect and does not affect the patient's health -related quality of life. The above results not only further support Oshitinib to become a better auxiliary treatment plan for patients with EGFR mutation positive and completely resection. The follow -up data has more expectations.
Adaura research update appeared at 2022 ESMO
In this most prestigious and influential oncology conference in Europe, Adaura studied not to live up to expectations, and the efficacy of longer follow -up time (nearly 4 years) and longer medication time (nearly 3 years) returned. The
According to the latest efficacy data after 44.2 months of follow-up time, among the completely removed NSCLC subjects with a completely removed II-IIIA NSCLC, the risk of recurring or death of the tumor of the tumor of Oshitinib with the placebo is compared with the placebo. Reduce 77%(Figure 1); among the completely removed IB-IIIA phase NSCLC subjects, the risk of recurring or death of the tumor recurrence or death of the subject taking Oshitinini with a placebo is 73%compared with the placebo (Figure 2) Essence It is worth mentioning that despite the longer follow -up time, the sub -groups still show significant benefits (Figure 3);
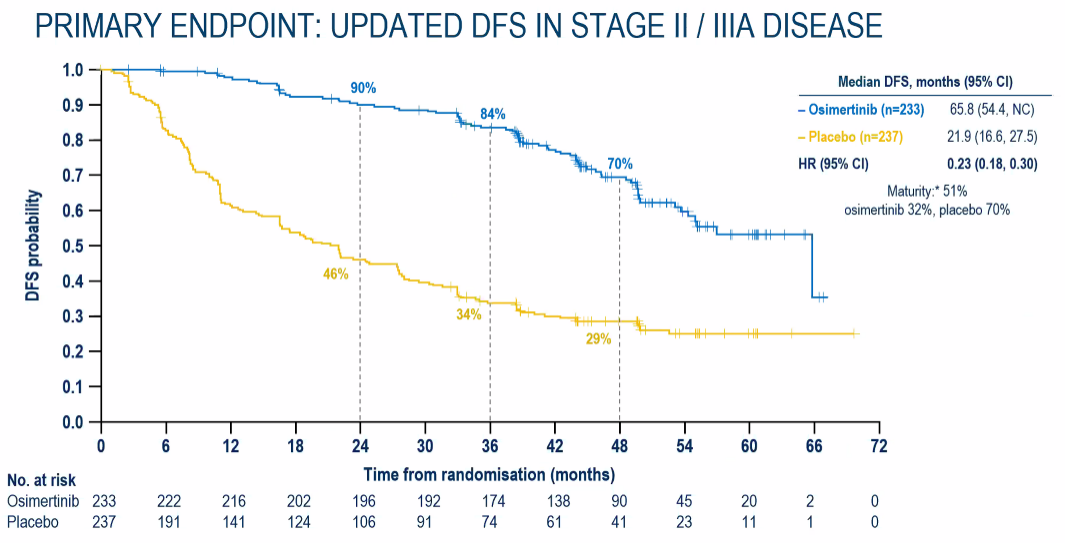
Figure 1. Main ending: II-IIIA Phase NSCLC patients have followed up DFS data for nearly 4 years
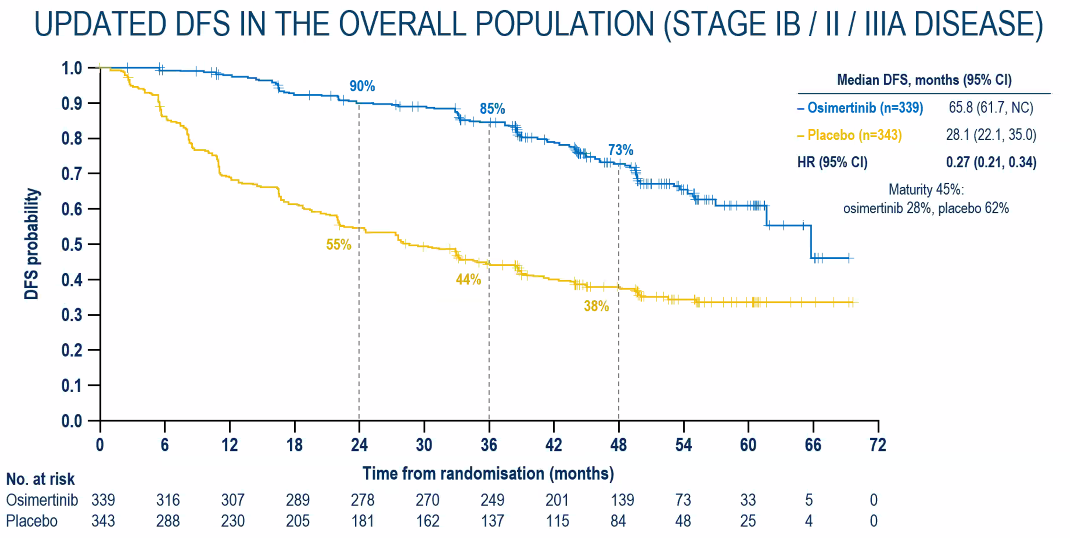
Figure 2. Key secondary endpoint: IB-IIIA Phase NSCLC patients with DFS data for nearly 4 years follow-up
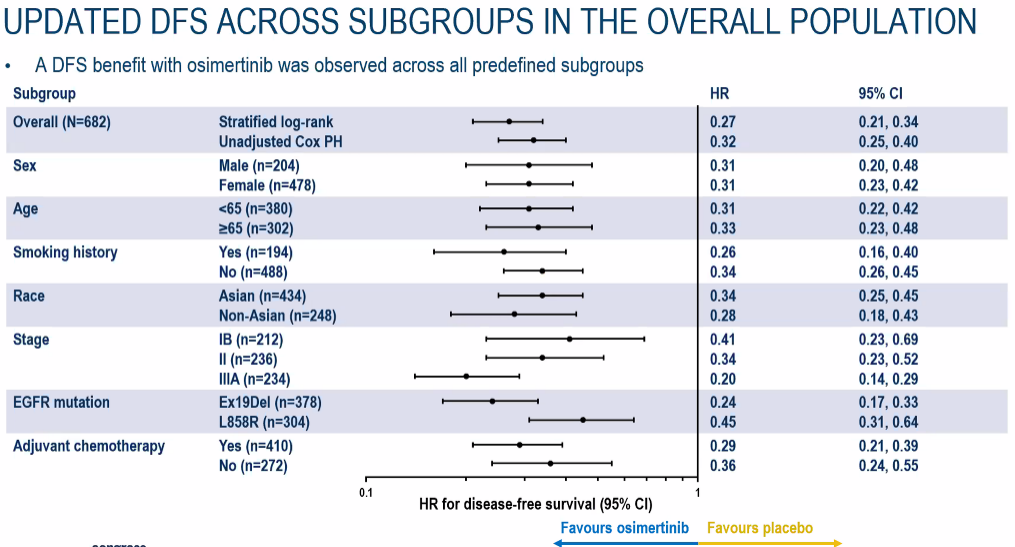
Figure 3. The DFS benefits of each sub -group in each of the follow -up time (including Phase IB)
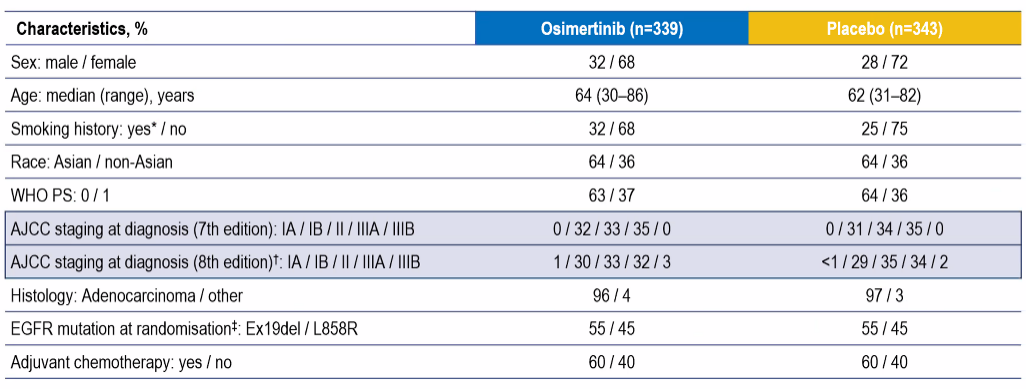
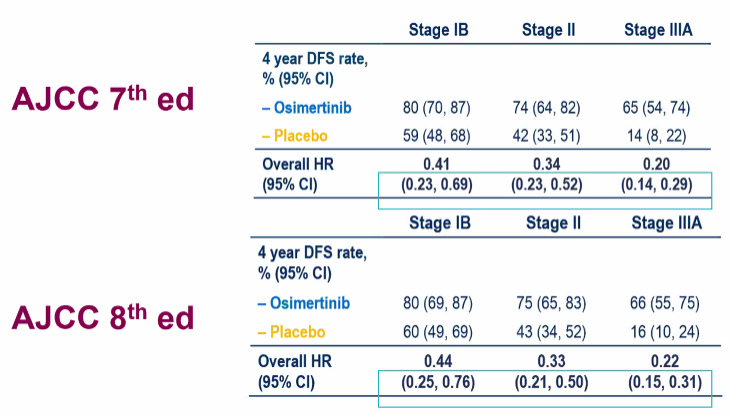
In addition, the research also explored the effects of different AJCC installment standards (AJCC 7 and 8 edition) on DFS data. It is found that each staging is not only similar to the two standards in terms of the proportion of the crowd, but also similar to the benefits of DFS. Essence Taking the IB stage as an example, the recurrence and death risk after nearly 4 years of follow -up decreased by 59%(AJCC 7th edition) vs.56%(AJCC 8th edition). Based on the 7th edition of AJCC or based on the 8th edition of AJCC, patients in each stage of IB-IIIA can achieve significant DFS benefits through Oshitininib (Figure 4); Figure 4. Different AJCC installment standards Population ratio and DFS data
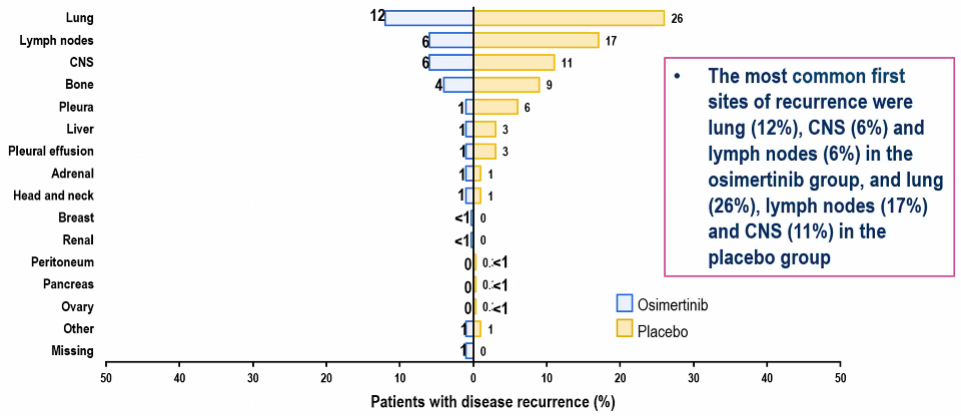
Among all the completely removed NSCLC subjects, only 6%of the subjects taking Oshitinib recurred, while the value of the placebo group was 11%, and the CNS DFS HR reached 0.24 (95%CI: 0.14 -0.42) (Figure 5).
Figure 5. The recurrence rate of different parts after long -term follow -up
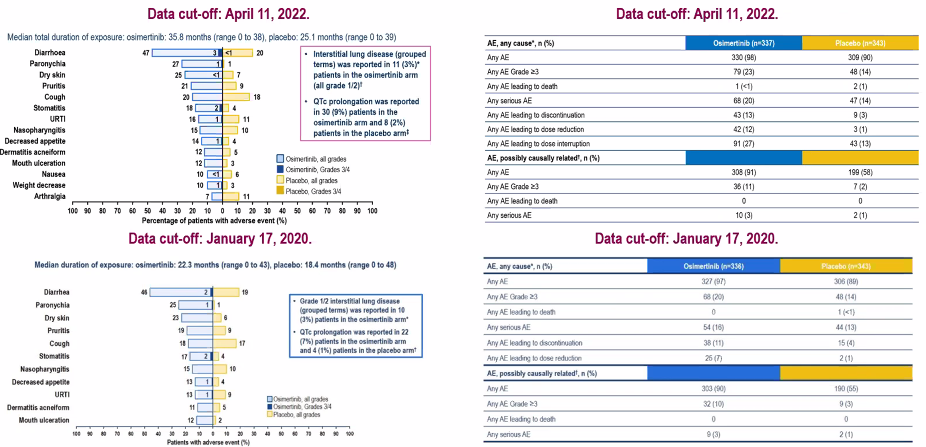
In addition, although the mid -range exposure time of Oshitinib in 35.8 months, the safety data of the patient is still consistent with the previous diarrhea. No new safety signal (≥3 -level treatment related adverse reactions are 11%. vs. past 10%) (Figure 6).
Figure 6. Safety data of different median exposure and follow -up time
Olinibic auxiliary therapy leading and looking at
How to prolong the total survival time of patients and the greatest benefit of patients is the research direction of lung cancer surgery. The Adauura update data released by the conference shows that after a long time of treatment and follow-up, patients in each stage of IB-IIIA can still achieve significant and long-lasting DFS benefits and consistent security of the past. Regardless of the 7th edition of AJCC or the 8th edition of AJCC. Osicinib refreshed the clinical practice of early EGFR mutations positive NSCLC auxiliary therapy, which is of great significance. At present, Oshitinib assist therapy has become a recognized standard treatment plan for the crowd and is the top in the field. I believe that we can see the efficacy and safety brought about by the auxiliary therapy of Oshitinib in the future. Benefiting, let us look forward to Adaura to study the wonderful appearance of the next time!
Expert Introduction
Wu Yilong

Oncology professor, doctoral supervisor, IASLC Outstanding Science Award winner
President of the Guangdong Medical Association (GDMDA)
Chief Expert of Guangdong Provincial People's Hospital (GDPH)
Director of Honorary Director of the Institute of Lung Cancer (GLCI)
Chairman of Chinese chest tumor research collaboration group (CTONG)
2018-2021 clinical medicine Global highly cited scientists in the world
The Chairman of the World Lung Cancer Conference (WCLC) in 2020
Former Chairman of the China Clinical Oncology Society, the chairman of the guidance committee
references:
[1] HERBST, ET Al.osimertinib as adjuvant therapy in patitions (PTS) with Stage IB – IIIIA EGFR Mutation Position (EGFRM) NSCLC AFTER COMPLETE TUMOR Resection: Adauura.2020 Asco, 1020 Asco, 1020 Asco, 1020 Asco.
[2]Tsuboi M,et al.Osimertinib as adjuvant therapy in patients(pts)with resected EGFR-mutated(EGFRm)stage IB–IIIA non-small cell lung cancer(NSCLC):updated results from ADAURA.2022 ESMO,LBA 47 Then, then, then
[3] Wu, TSuboi et al.n English; 383: 1711–1723.
[4]Koch AL,FDA Approval Summary:Osimertinib for Adjuvant Treatment of Surgically Resected Non-Small Cell Lung Cancer,a Collaborative Project Orbis Review.Clin Cancer Res.2021 Dec 15;27(24):6638-6643.
[5] Sulfate Oxidic Nini Tablets (version 2021.04.07).
[6] wu yl, et al.Postopetive Chemotherapy Use and Outcomes from Adauura: Osimrtinib as adjuvant therapy for resed egfr multived nsclc.2020 WCLC.
) With Resected EGFR Mutated (EGFRM) NSCLC (Adauura): Central Nervous System (CNS) Disease Recurrence.2020 ESMO, LBA 1.
Approval number: CN-102711
This article's interview/writing/release is supported by Astraikon for reference only for medical and health professionals, not for promoting purposes.
Source: Medical Community
School pair: Zang Hengjia
Responsible editor: Tian Dongliang
Hot text recommendation
- END -
Liqiu 丨 older half of the year, hello autumn

On August 7, 2022, the first solar term in autumn was ushered in: Lili Autumn.Sinc...
For employment, please pay attention!

Li Keqiang presided over the executive meeting of the State CouncilPolicies and me...