2022 Medical Insurance Drug Catalog Adjustment: 344 drugs passed the preliminary form review
Author:Hebei Radio and Television Sta Time:2022.09.07
Interpretation of the public list of the drug list
According to the adjustment of national basic medical insurance, work injury insurance, and maternity insurance drugs (hereinafter referred to as the national medical insurance drug catalog) in 2022, the State Medical Insurance Administration recently conducted preliminary review of the application drugs, and conducted the examination of drugs and information that passed the review. Public announcement. The interpretation of the work is as follows.
First, what are preliminary form review?
The preliminary form review is preliminary review of whether the application of drugs and the adjustment of the national medical insurance and drug catalog and the integrity of the drug information of the national medical insurance drug.
In accordance with the "Interim Measures for the Management of Basic Medical Insurance Drugs", the national medical insurance and drug catalog adjustment is implemented to implement an enterprise application system. The National Medical Insurance Administration adjusted the work plan of the national medical insurance and drug catalogs to determine the application conditions and requirements, and each declared subject voluntarily declared. For preliminary review of drug declaration materials, on the one hand, the drugs that can be declared to meet the application conditions, on the other hand, review the integrity and standardization of the application information, and verify the authenticity of some materials according to the work needs of the work. It is conducive to ensuring that the information provided to experts is more accurate and complete. At the same time, in order to take the initiative to accept social supervision and ensure accurate formal review results, we publicize the medicines and parts of the medicines and some information through preliminary form review results. We welcome all sectors of society to put forward valuable opinions and suggestions. Considering that the economy of drugs mostly involves the company's secrets and core interests, the information related to the economy's application information submitted by the enterprise has not been announced.
2. Does the preliminary form review mean that it has been included in the national medical insurance and drug catalogs?
In accordance with the "Interim Measures for the Management of Basic Medical Insurance Drugs" and "Temporary State Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug Catalog" (hereinafter referred to as the "Work Plan"), the medical insurance catalog is divided into enterprise declaration and form review , Expert review, negotiation bidding, etc., formal review is only one of them. Through form review, the drug is eligible to enter the next expert review session. Only by successfully adjusting the directory adjustment can it be eventually included in the national medical insurance directory.
3. What is the changes compared with the preliminary form review of the national medical insurance category adjustment in 2022?
From 9:00 on July 1, 2022 to 17:00 on July 14, 2022, the national medical insurance information platform received a total of 537 corporate declaration information, involving 490 drugs (universal names, the same below). After review, 344 drugs passed the preliminary review, with a proportion of 70%. Compared with 2021 (274 drugs passed) in 2021, the number of drugs for preliminary form review has increased. In terms of pass rate, the proportion of drugs outside the directory is 60%, and the proportion of drugs in the directory is 91%.
According to the "Work Plan", this year's review method and review process have undergone some changes. Therefore, we further optimized the content of the enterprise declaration and further enriched the requirements for the application of the drug in the application information, including effectiveness, safety, and, and Economics, innovation and fairness, etc., and ask companies to submit drug information abstract slides, giving enterprises more opportunities to introduce their own varieties to experts.
Fourth, how do you view some current market prices that are expensive to pass preliminary review?
Since its establishment, the National Medical Insurance Bureau has resolutely implemented the decision -making and deployment of the Party Central Committee and the State Council, and has always firmly grasped the functional positioning of the basic medical insurance "guarantee basic", insisted on doing its best, doing it, and determining the scope of guarantee; , Taking the affordability of the medical insurance fund and the participating masses as the basis for the adjustment of the directory, the prices of exclusive drugs are greatly reduced through admission negotiations and other methods; the balance between the basic medical needs and clinical technological progress of the masses is always focused. Maintain fairness.
This year, some expensive drugs have passed the preliminary review of the preliminary form, which only said that the drug meets the application conditions and has obtained the qualification to enter the next link. Whether this type of medicine can eventually enter the national medical insurance drug catalog also needs to be strictly evaluated in many ways to include economics. The exclusive drugs that pass the review must be negotiated and the non -exclusive drugs must be bidded. Essence
5. What are the key considerations for the treatment of new crown pneumonia?
In order to do a good job of preventing and controlling the new crown epidemic, the National Medical Insurance Bureau adheres to the "people -centered people" and focuses on the overall situation of the epidemic prevention and control. The "two guarantees" policies of treatment "temporarily incorporate relevant drugs into the scope of medical insurance payment, which provides strong support for the prevention and control of the epidemic.
In the context of the normalization of the epidemic prevention and control, from the first implementation of the independent application system of the medical insurance drug directory enterprise in 2020, the State Medical Insurance Administration attaches great importance to the treatment of new crown pneumonia. Including the "new type of coronary virus pneumonia diagnosis and treatment plan "(Hereinafter referred to as the" Diagnosis and Treatment Plan ")") ")") ") Pharmaceuticals") "") ")") ")") Pharmaceuticals ")" ")") ")") ") Pharmaceuticals") "") ")") ")") Pharmaceuticals ") as one of the application conditions. A number of new crown therapy medication has been included in the medical insurance catalog.
This year, the ninth edition of the "diagnosis and treatment plan" included a group of medicines just listed in my country.During this application, some medicines conducted independent declarations and passed preliminary examinations. Some medicines did not declare, and we respect the choice of enterprises.For drugs that have been declared and reviewed in form, we will carry out follow -up work in accordance with the procedures, and strive to officially include the medical insurance directory at a reasonable price.6. How will this work after this publicity?
In the next step, we will further verify the relevant information based on the feedback received during the publicity period, determine the scope of the drug that finally passes the formal review, and announce it to the society.Subsequently, we will promote the follow -up work of expert review and negotiation bidding.
Site screenshot


- END -
4 public kindergartens, primary and secondary schools in Luolong District, unveiled and put -in

On August 30, the Lulong District First Experimental Primary School Exhibition Roa...
From today, you can choose to deliver the delivery in various ways
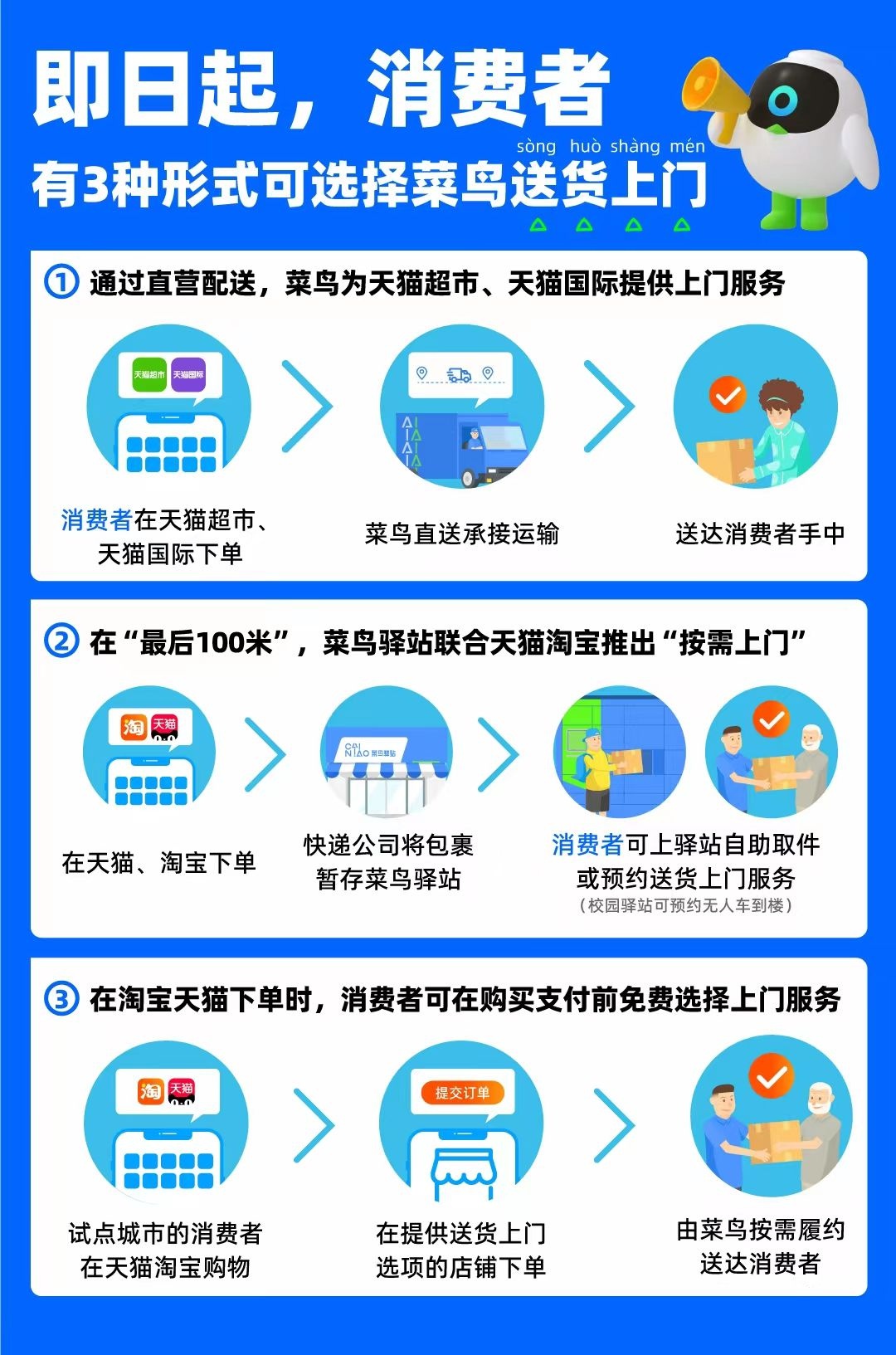
The Yangtze River Daily Da Wuhan Client July 26 (Reporter Zhang Yue) New trends ar...