It will be implemented from December!These medicines cannot be sold online
Author:Shanghai Yangpu Time:2022.09.03
Recently, the General Administration of Market Supervision issued the "Measures for the Supervision and Administration of Drug Network Sales" and implemented from December 1, 2022. The "Measures" stipulate the drug network sales management, platform responsibility performance, supervision and inspection measures and legal responsibilities. The main contents include:
Clarify the qualifications and requirements of the pharmaceutical business enterprise engaged in drug network sales, and clarify the vaccine, blood products, anesthesia drugs, psychotropic drugs, medical drugs, radioactive drugs, pharmaceutical and chemicals and other countries in accordance with the law Do not sell on the Internet.
Clarify that third -party platforms should set up drug quality and safety management institutions, equipped with pharmacy and technical personnel, and establish and implement drug quality and safety, pharmaceutical information display, prescription review, prescription drug real -name purchase, drug distribution, transaction record preservation, adverse reaction report, complaint report processing, etc. Management system and record in accordance with regulations. The platform is required to sign an agreement with the pharmaceutical network sales enterprise to clarify the responsibility for the quality and safety of the pharmaceutical quality of both parties. It stipulates that the platform shall perform the obligations and reports of the stop service and report of the discovery of serious violations of the law, and strengthen the platform And the coordination obligation in supervision and inspection.
Considering the safety risks of medication and consistency management requirements for online and offline, clearly implement a real -name system for the sales of prescription drug networks, and conduct prescription review and deployment in accordance with regulations.
The prescription drugs and non -prescription drugs should be distinguished, and information on the homepage of prescription drugs and the homepage shall not directly display the packaging, labels and other information on the homepage.
Before the prescription review, no information such as the manual shall be displayed, and the relevant services of the prescription medicine purchase shall not be provided.
Before the preparation of prescription drugs, it shall fully inform consumers to tell the relevant risk warning information and confirm the knowledge by consumers.
The corresponding legal liability for the illegal behavior of the drug network sales is according to law. To strengthen the control of drug safety risks, if there is a hidden safety hazard in the evidence, it is clear that the drug regulatory authorities can adopt advice, interviews, rectification within a time limit, and suspending production, sales, use, and imports.
Edit: Zhang Tianyi
Data: CCTV News Xinhua News Agency
*Reprinted, please indicate the official WeChat from Shanghai Yangpu
- END -
The Guangdong Provincial Department of Education reported 3 illegal training and investigation situation
Sun Wei, a reporter from Yangcheng Evening News: Recently, the Guangdong Provincial Department of Education issued the Notice on the Investigation and Investigation of Disciplinary Training Behaviors
Pay attention to prevent!The strongest rainfall since the north will now enter the flood
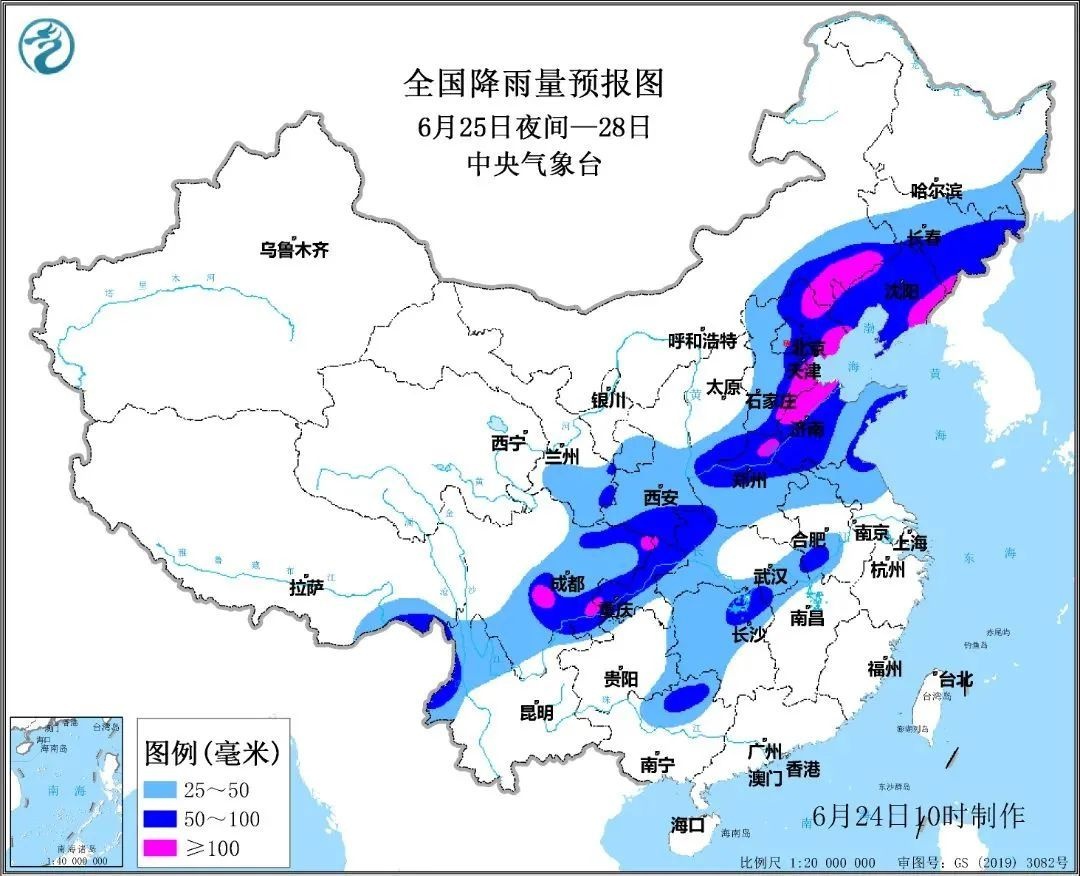
The latest forecast of the Central Meteorological Observatory, from the night of J...