ASCO Breast Cancer Guide Update: HER2 Low Expressing breast cancer ushered in a new plan!
Author:Cancer Channel of the Medical Time:2022.08.29
*For medical professionals for reading reference
Come and learn!
In 2021, ASCO released chemotherapy and targeted treatment guidelines for patients with epidermal growth factor receptor 2 (HER2) negative metastatic breast cancer. Since then, the guide will be updated again on August 4, 2022. The rapid update of the new version of the guide is now interpreted as follows:

Screenshot of the guide

Scan the two -dimensional code above and get more tumor guidelines information
DS8201
Can be used for HER2 low expression/positive metastatic breast cancer
Guide update suggestion: HER2 IHC 1+/IHC 2+ and ISH negative metastatic breast cancer patients who have received at least first -line metastatic disease chemotherapy in the past, and those with hormone receptor and ineffective endocrine therapy should use DS8201 (based on Evidence -based evidence is greater than disadvantages; evidence quality: medium; recommendation intensity: strong recommendation).
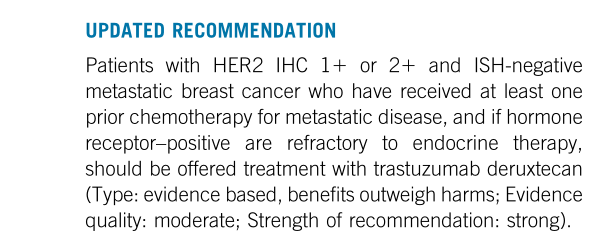
Guide update recommendation part
Among the breast cancer without HER2 amplification, excessive expression, or both above, about 60%of patients are manifested as low -level expression of HER2 (defined as IHC 1+/IHC 2+ and ISH negative) [1]. The existing HER2 targeted therapy does not effectively improve the clinical ending of HER2 low expression of breast cancer patients [2]. The update of this guide marks new hope for HER2 low -expression metastatic breast cancer patients.
Study interpretation
Recommended evidence about DS8201 in the guide is based on Destiny-Breast04. This research is a clinical trial of a phase III, two groups, open labels, and random centers:
Study and publish screenshots
▌The trial plan
• Study in patients with low expression metastatic breast cancer who have previously accepted first -line or second -tier chemotherapy, randomly allocated at a ratio of 2: 1 to the chemotherapy group (TPC, which accepts DS8201 or doctors. Bollinger, Jesitabin, paclitaxel and albumin).
• The main ending indicators are the non -progressive survival period (PFS) of the hormone receptor positive queue (PFS). The key secondary ending indicators are the PFS of all patients. Other ending indicators include hormone receptor positive queues and the total survival period of all patients (OS). Objective relief rate (ORR), relief duration (DOR) and safety.
Script
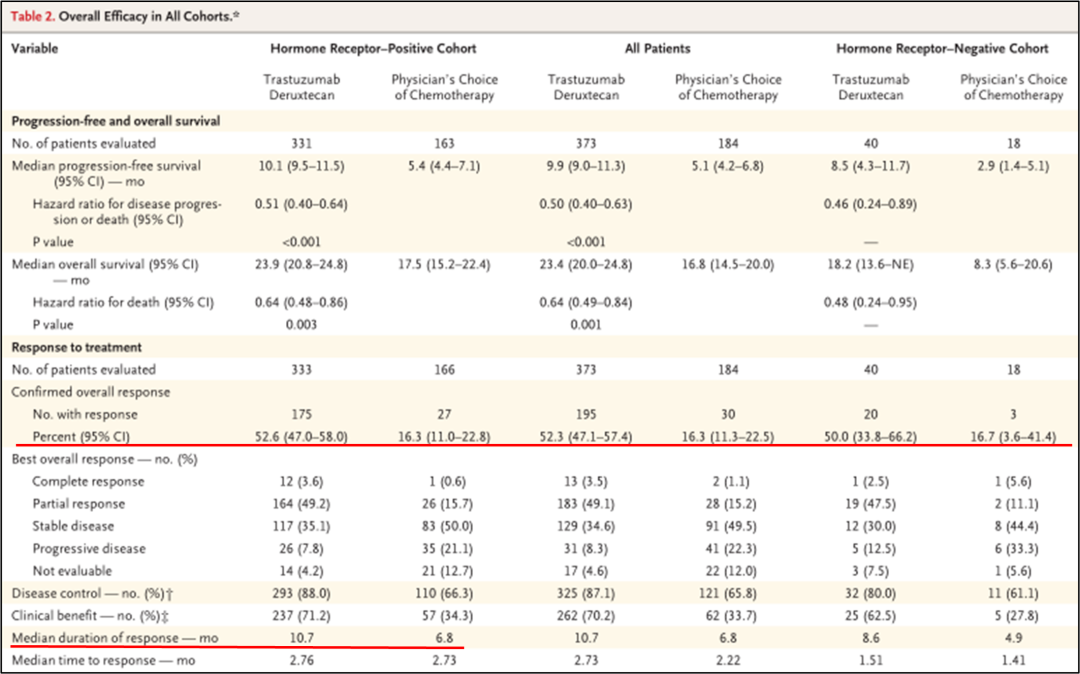
In the end, 494 patients with positive hormone receptors were included, and 63 patients with hormonal receptor -negative metastatic breast cancer patients. During the mid -range follow -up period of 18.4 months, the mid -level treatment duration of the DS8201 group was 8.2 months, and the TPC group was as a group of TPC groups. 3.5 months.
• The research results show that in the positive queue of hormone receptor:
DS8201组的中位PFS为10.1个月(95%CI 9.5-11.5),而TPC组为5.4个月(95%CI 4.4-7.1)(HR 0.51;95%CI 0.40-0.64;P<0.001), See Figure 1A.
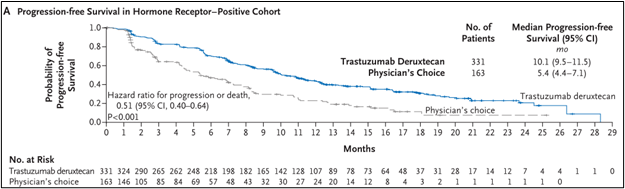
Figure 1A
The median OS of the DS8201 group is 23.9 months (95%CI 20.8-24.8), and the TPC group is 17.5 months (95%CI 15.2-22.4) (HR 0.64; 95%CI 0.48-0.86; P = 0.003), see Figure 1c.
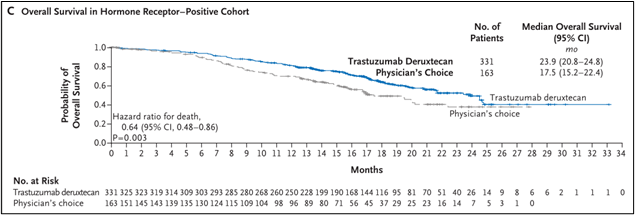
Figure 1C
• Among all patients including hormone receptor and receptor -negative:
The median PFS of the DS8201 group is 9.9 months (95%CI 9.0-11.3), and the TPC group is 5.1 months (95%CI 4.2-6.8) (HR 0.5; 95%CI 0.40-0.63; P <0.001), see Figure 1B.

Figure 1B
The median OS of the DS8201 group is 23.4 months (95%CI 20.0-24.8), and the TPC group is 16.8 months (95%CI 14.5-20.0) (HR 0.64; 95%CI 0.49-0.84; P = 0.001), see Figure 1d.
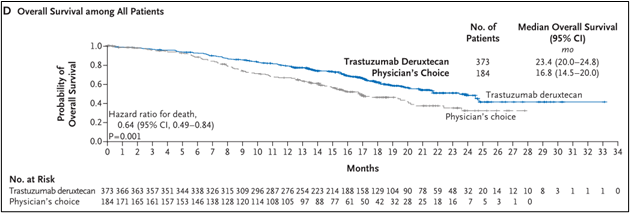
Figure 1D
• In a hormone -receptor -negative queue:
The median PFS of the DS8201 group is 8.5 months (95%CI 4.3-11.7), and the TPC group is 2.9 months (95%CI 1.4-5.1) (HR 0.46; 95%CI 0.24-0.89). See Figure 2-1 Essence
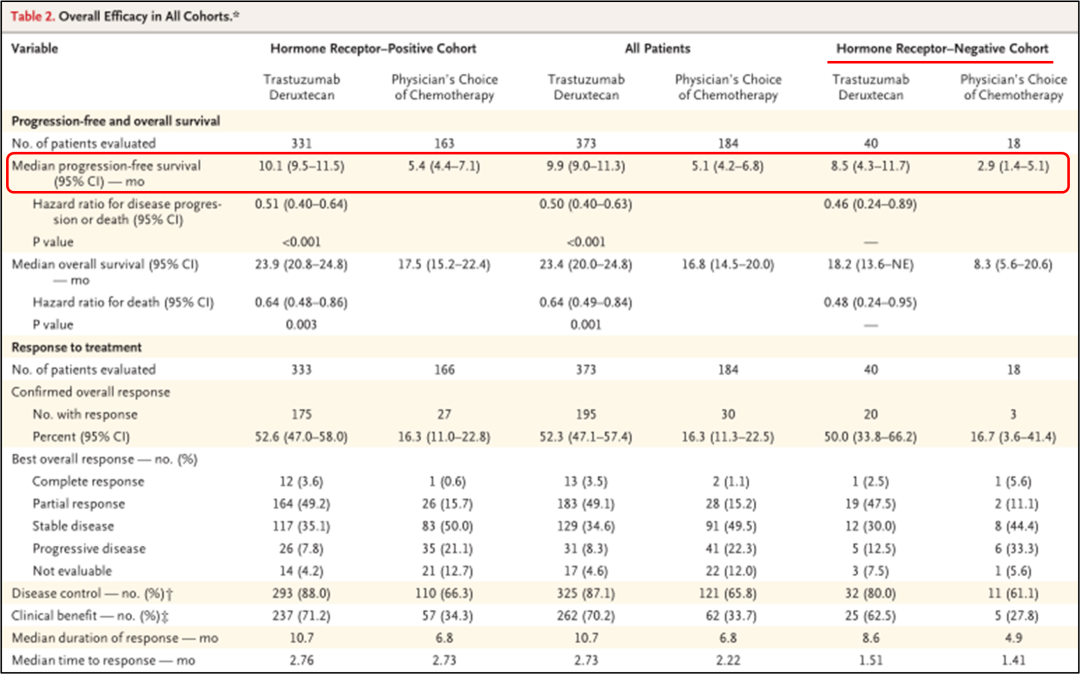
Figure 2-1
The median OS of the DS8201 group is 18.2 months (95%CI 13.6- is not evaluated), and the TPC group is 8.3 months (95%CI 5.6-20.6) (HR 0.48; 95%CI 0.24-0.95). 2.
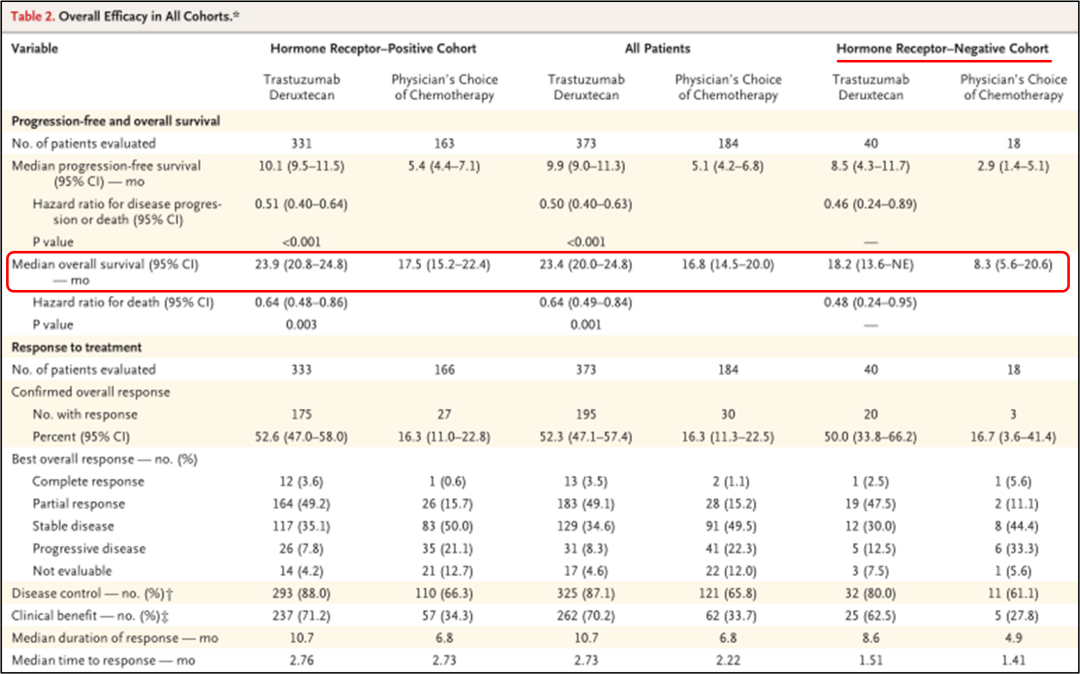
Figure 2-2
: Other ending indicators:
• In the positive hormone receptor queue, the ORR of the DS8201 group is 52.6%(95%CI 47.0%-58.0%), and 16.3%(95%CI 11.0%-22.8%) in the TPC group, and the median DOR respectively For 10.7 months and 6.8 months;
• Among all patients, the ORR percentage of the two groups was 52.3%(95%CI 47.1%-57.4%) and 16.3%(95%CI 11.3%-22.5%);
• In the hormone receptor negative queue, the percentage of the two groups of ORR was 50.0%(95%CI 33.8%-66.2%) and 16.7%(95%CI 3.6%-41.4%), as shown in Figure 2-3. Figure 2-3
▌ safety
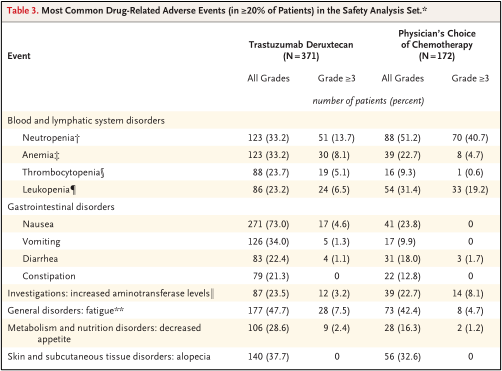
• Safety analysis set included 371 patients in the DS8201 group and 172 patients in TPC groups. The severe adverse reaction rate of the DS8201 group was 27.8%, the TPC group was 25.0%, and the incidence of adverse events in levels 3 and above was 52.6%and 67.4%.
• 14 patients who received DS8201 treatment had drug -related deaths, including the cause of death, including pneumonia, ischemic colitis, DIC, dyspnea, fever neutrophils and sepsis; TPC group No related drug death occurred.
• In the DS8201 group, the most common drug adverse reactions include nausea (73%of patients), fatigue (47.7%of patients) and hair loss, and these are more frequent than the TPC group (23.8%, 42.4%, respectively And 32.6%), see Table 3.
table 3
Destiny-Breast04 represents the first clinical random III test for Her2 IHC 1+ or 2+ and ISH negative metastatic breast cancer patients. For non -targeted therapy. Compared with the TPC group, regardless of the state of hormone receptor (positive or negative), the risk of disease progress or death risk of patients with DS8201 has decreased by about 50%, and the risk of death is reduced by about 36%.
In the past, HER2 targeted therapy significantly improved the clinical ending of HER2-positive (defined as IHC 3+/IHC 2+ and ISH positive) breast cancer patients, but HER2 low expression patients did not obviously benefit from it [2-3]. The ASCO guideline update shows that the activity of DS8201 is better than standard chemotherapy for patients with advanced breast cancer in HER2, which highlights the clinical significance of DS8201 for the population of patients in HER2.
Due to the smaller hormone receptor -negative queue in this study, further test research is still required to discuss the reasonable application of drugs in this type of patients. In addition, interstitial pulmonary disease or pneumonia is an important risk related to DS8201, and the understanding and research of its risk factors are still in progress [4].
references:
[1] SCHETTINI F, Chic N, Brasó-Maristany F, ET Al.Clinical, Pathology, and PAM50 Gene Expression Features of her2-LOW Breast Cancer.npj Breast Cancer 2021; 7: 1-1.
[2]Fehrenbacher L,Cecchini RS,Geyer CE Jr,et al.NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1 +OR 2.J Clin Oncol 2020; 38: 444-453.
[3]Burris HA III,Rugo HS,Vukelja SJ,et al.Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2(HER2)-positive breast cancer after prior HER2-directed therapy .J clin oncol 2011; 29: 398-405.
[4] MODI S, Jacot W, Yamashita T, et alstuzumab deruxtecan in Previously Treated Her2-Low Advanced Breast Cancer.n English .Epub 2022 jun 5.pmid: 35665782.
The first release of this article: the medical world tumor channel
Author of this article: I want
Review of this article: Xu Weiran Editor: Sweet
- END -
pay tribute!Persevere at high temperature

Zhong Jingtao/Photo Haidian Transportation Detachment District Garden Greening Bur...
Institutional Squadron Combat: Comprehensively test the true kung fu of fire extinguishing rescue
In the past few days, the Aba Forest Fire Detachment has concentrated on the three...