ASCO Guide Recommended Lung Cancer Treatment!Drive gene -negative IV stage adds options
Author:Cancer Channel of the Medical Time:2022.08.11
*For medical professionals for reading reference
2022 ASCO Drives Gene negative IV Lung Cancer Treatment Guide Update
As the diagnosis and treatment of lung cancer enters the targeted era, multiple genes that have played a key role in the process of non -small cell lung cancer (NSCLC) disease are available. (ALK) Patients with late -stage NSCLC with positive genetic positive genes, most targeted drug treatment can effectively prolong patients' survival (OS) and improve the quality of life of patients.
In the past few decades, patients with late NSCLC who driven gene negative can only use traditional mortar chemotherapy for treatment. In recent years, with the application of immune drugs, more and more patients expressed by PD-L1 have benefited from immunotherapy, which has also gradually diversified the treatment methods of these patients. For patients with advanced NSCLC for driving gene negative, the choice of immunohist drugs or immune combined therapy is currently based on the expression of the patient's PD-L1.
Recently, the United States Clinical Oncology Society (ASCO) has updated the clinical practice guidelines for the phase IV NSCLC system therapy. This update mainly points to the effectiveness of the first, second, and third -line therapy of patients with phase IV NSCLC patients driving gene negative. Among them, the first-line therapy for squamous carcinoma (SCC) patients or non-SCC patients with different PD-L1 expression [2].

Figure 1 Guide to post screenshots
Lung squamous carcinoma, pulmonary adenocarcinoma, different expression levels of PD-L1, different first-line therapy schemes!
1
PD-L1 high-expressed non-SCC patients
▌ Update points:
New recommendation 1.5, 1.6
For non-SCC patients with PD-L1 high expression (TPS ≥ 50%) and PS 0-1, clinicians can provide single-drug Adi Zhuabi and Simi Priley monoclonal treatment (recommendation intensity: high).
Added recommendation 1.7
For non-SCC patients with PD-L1 high expression (TPS ≥ 50%) and PS 0-1, clinicians can provide single-use Nawuli Mipida and Ipmu Mipida, or Nawuli Mipida Single antibody combination of platinum -containing chemotherapy (Recommended intensity: low).
Delete the original recommendation 1.5
No longer recommend the application of other examination point inhibitors and the application of combined chemotherapy.
▌ Related research:
In the IMPOWER110 test, compared with the chemotherapy group, patients with high PDL1 high -expression OS were significantly extended (20.2 months vs13.1 months) after receiving Ayidzab's treatment [3].
In the EMPOWER-LUNG phase III test, patients with PD-L1 TPS ≥50%, smoking history and driving genetically negative patients use the efficacy of Simi Prilum Mipido and chemotherapy. The latest data of the study shows that patients receiving Simi Priley monoclonal antibodies have obvious OS and PFS advantages [4].
2
PD-L1 low expression or negative non-SCC patient
▌ Update points:
Added recommended 2.7
For non-SCC patients with PD-L1 low expression (TPS 1%-49%) or negative (TPS 0%), PS 0-1, clinicians can provide single-use Nawuli Mippitarian and Ipmu Mupage, or Nawuli Mipida and Ipmu Mipida combined with two cycles of platinum -containing chemotherapy (recommendation intensity: low).
▌ Related research:
Based on the results of the CheckMate 227 and CheckMate 9LA tests, compared with the simple chemotherapy, Nawuli Mippitive+Ipmu Mupage (17.2 months vs12.2 months) and Nawuli Mipida+Ipmu Microex+chemotherapy (16.8 months VS9.8 months) can significantly extend the OS of PD-L1 to express negative patients [5,6].
In the CheckMate 9LA trial, Nawuli Mippochemical+Ipmu Mipoid+chemotherapy scheme can be used for PD-L1 low expression patients, but the combination of three drugs in this scheme is complicated, high risk, and uncertain efficacy, so the recommendation intensity is low to be low. Essence
3
PD-L1 high-expressed SCC patient
▌ Update points:
New recommendation 3.3, 3.4
For patients with PD-L1 high expression (TPS ≥ 50%) and PS 0-1, clinicians can provide single-drug Adi Zhuabi and Simi Pribei monoclonal treatment (recommendation intensity: high).
New recommendation 3.5
For patients with PD-L1 high expression (TPS ≥ 50%) and PS 0-1, clinicians can provide single-use Nawuli Mipida and Ipmu Mipida, or Nawuli Mipida Platinum chemotherapy (recommended intensity: low) with 2 cycles.
Delete the original recommendation 3.3
No longer recommend the application of other examination point inhibitors and the application of combined chemotherapy.
4
PD-L1 low expression or negative SCC patient
▌ Update points:
New recommendation 4.5
对于PD-L1低表达(TPS 1%-49%)或阴性(TPS 0%)、PS 0-1的SCC患者,临床医生可提供单用纳武利尤单抗和伊匹木单抗,或纳Wudi Yumab and Ipmu Mipido combined with two cycles of platinum -containing chemotherapy (Recommended intensity: low).
Summary of the first line therapy
KEYeTE-024, Impower110 and EMPOWER-LUNG 1 test data show that NSCLC patients with PD-L1 high expression (TPS> 50%) can benefit from anti-PD-1 or anti-PD-L1 antibody single drugs. The ORR of the chemotherapy combined immunotherapy group is higher than the anti-PD-1/PD-L1 antibody single drug treatment group. Although chemotherapy combined immunotherapy does not guarantee the rapid reduction of tumor cells, compared with immunotherapy treatment, combined treatment can slow the decline in early OS in patients.
In summary, in the first-line treatment plan recommended by the guide, for PD-L1 high-expressed SCC and non-SCC patients, except for PD-L1 inhibitors that contain previously used or combined chemotherapy PD-L1 inhibitors (K In addition, the treatment scheme of the PD-L1 inhibitors Aidaridumab (T drug) and the Simi Prilum monoclonal treatment alone will be used as a strong recommendation.
What are the most effective second and third -line therapies?
▌ Update points:
New recommendation 5.1
For non -SCC patients who have received ICI and chemotherapy, clinicians can provide the treatment of paclitaxel combined with Bevarzab (Recommended intensity: low) in the second -tier treatment.
▌ Update points:
Added recommendation 6.1
For non -SCC patients who have undergone chemotherapy combination or non -SCC patients who have undergone chemotherapy combination or non -SCC treatment for ICI, clinicians can provide single -medicine Pemeuscese or Dorcatsen or paclitaxel in third -line treatment. The choice of combined with Bevarzumab (Recommended intensity: low).
Two or three -line therapy summary
For most patients with gene negative IV NSCLC, first -line treatment (platinum dual pharmaceutical chemotherapy and immunotherapy combination) The treatment options for progress or recurrence usually include single drug chemotherapy and different drugs. Dorcio (all histological types), Permese (Non -SCC NSCLC), and the weekly paclitaxel combined with Beday Mipopolymal (Non -SCC NSCLC) can become the choice of second- and third -line therapy.
In summary, in the second and third -tier treatment plan recommended by the guide, for patients with initial treatment are not patients with chemotherapy combined with immunotherapy, they should undergo an early treatment, that is, platinum dual pharmaceutical chemotherapy (if initial treatment is single medicine treatment combined with ICI ) And immunotherapy (if initial treatment is platinum dual pharmaceutical chemotherapy).
Figure 2 Drives the classification treatment and intensity recommendation of non -small cell cancer in stage of gene negative IV
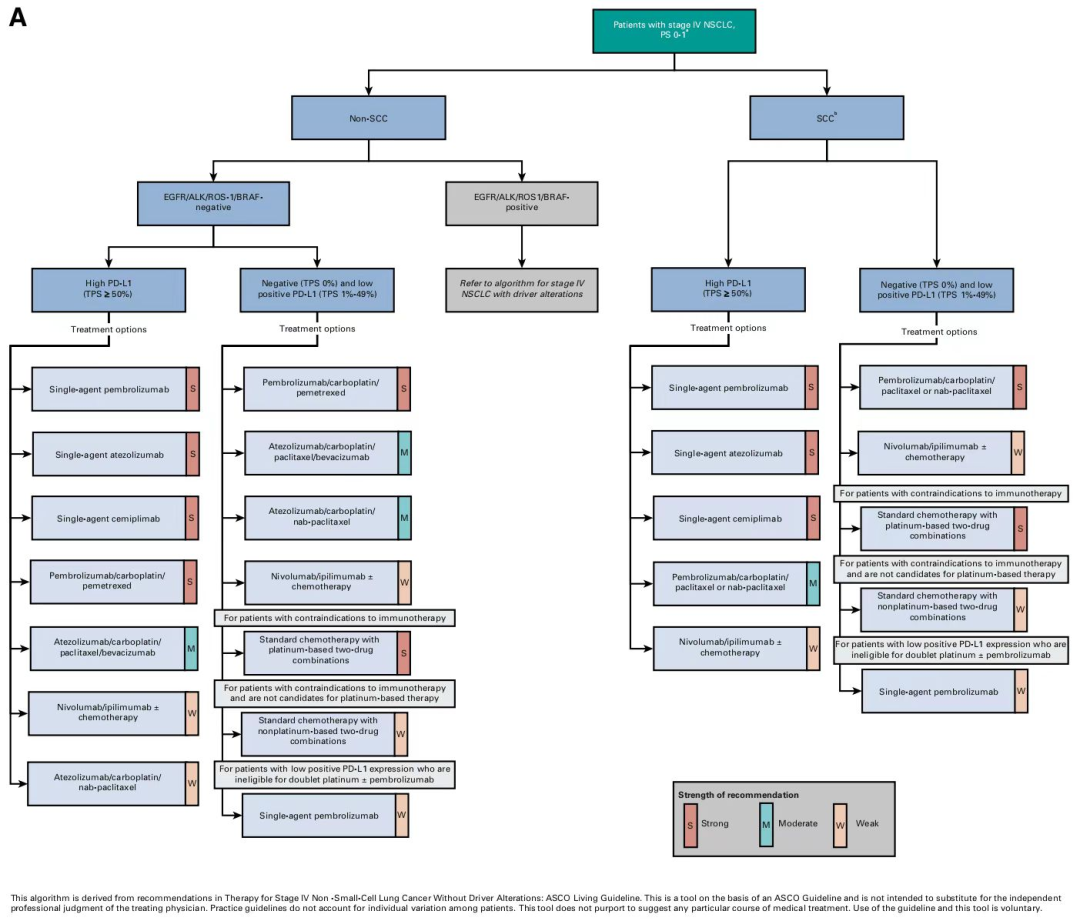
Summarize:
Anti-PD-1/PD-L1 antibody has become the cornerstone of front-line treatment. With the improvement of long-term follow-up data, the improvement of long-term survival rates is becoming more and more obvious.
Although the classification treatment and intensity recommendation of non -small cell lung cancer in stage of drive gene negative IV have been further refined, the guide needs to be optimized. For example, the OS phase of the Adi -Mile Microex is only a temporary research end, and it is not sure its impact on tumor load. For another example, in the CheckMate 9LA study, the asylum analysis expressed in the organization and PD-L1 is still indirect evidence. It is expected that the renewal of the guide in the future will make the precision treatment of the driving gene negative NSCLC to the next level.
references:
[1] Yuan M, Huang L-L, Chen J-H, Et Al.The Emerging Treatment Landscape of Targeted Therapy in Non-Small-Cell LUNG CANCER.SIGNAL Transduction and Targeted Therapy, 2019,4 (1): 61.61.61.
[2]Singh N,Temin S,Baker S,Jr.,et al.Therapy for Stage IV Non-Small-Cell Lung Cancer Without Driver Alterations:ASCO Living Guideline.Journal of clinical oncology:official journal of the American Society of Clinical Oncology, 2022: JCO2200825.
[3]Rizvi NA,Cho BC,Reinmuth N,et al.Durvalumab With or Without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer:The MYSTIC Phase 3 Randomized Clinical Trial.JAMA oncology,2020, 6 (5): 661-674.
Controlled, PHASE 3 TRIAL.LANCET, 2019.
[6] Hellmann MD, Paz-Arts L, Bernabe Caro R, et al.Nivolumab Plus iPilimumab in Advanced Non – Small-Cell LUNG CANCER.NEW England Journal of Medicine, 2019,381: 2020-2031.
The first release of this article: the medical world tumor channel
Author of this article: Little catfish
Editor in charge: Sweet
- END -
Finally it's going to rain!At the end of July, Zigong will have mid -to -heavy rain in Sichuan.
Cover news reporter Liu Ke ShengOn July 10, Zigong Meteorological Observatory in Sichuan Province released the weather trend forecast in mid -July.It is expected that the average temperature in mid -J
230 billion yuan "financial tide" promotes the high -quality development of Xi'an's economy

Practice Rongyi——In our city holding to promote high -quality economic developme...