WCLC big coffee interpretation | Professor Zhou Cun: IMPOWER010 to study OS data for the first time, consolidating Adi Zhuabi as a standard auxiliary treatment plan!
Author:Medical community Time:2022.08.08
At this year's WCLC conference, IMPOWER010 studied the latest heavy OS data for assisted immunotherapy!
As the next "air outlet" of lung cancer immunotherapy, the application of PD-1/L1 inhibitors in early non-small cell lung cancer (NSCLC) patients in patients with perioperative treatment during surgical treatment has attracted much attention. OS, as a gold standard for evaluating the efficacy of anti -tumor drugs, has always been the focus of efficacy indicators for immune neo -assisted/auxiliary therapy research.
At the recently held 2022 World Lung Cancer Conference (WCLC 2022), as the first clinical III key research IMPOWER010 that confirmed the benefit of the benefit of the benefit of the auxiliary immunotherapy, it officially announced the preset first OS midterm analysis results. This is also the first time that the auxiliary immunotherapy Report OS related data. How to look at the significance of this blockbuster data and the impact on assisted immunotherapy? In this regard, the "medical community" invited one of Impower 010 researchers and Professor Zhou Cun, Shanghai Luopuke Hospital affiliated to Tongji University to interpret the latest IMPOWER010 research OS data and discuss the application prospects of assisting immunotherapy.
IMPOWER010 studies OS obvious benefits,
Auxiliary immunotherapy promises
IMPOWER010 studies the previously published data is mainly the results of the disease-free survival (DFS). Studies have shown that Ayidarzumab aid therapy for PD-L1 tumor cells (TC) expression Platinum-based patients with phase II-IIIA after the auxiliary chemotherapy have significant DFS benefits, and the risk of recurrence or death of patients with a decrease in the risk of death (DFS HR = 0.66, P = 0.004). 60%[1]. Based on the above data, Adi Zhuzabi has approved the auxiliary treatment certificate in China, the United States, and Japan.
Professor Zhou Cun said, "IMPOWER010 research, as an iconic clinical study of early NSCLC auxiliary immunotherapy, is an epoch -making study that has changed the study of clinical practice. As the first III study in III in the currently approved assisted immunotherapy indication indication indication indication indications , Adi Zhuzumab launched a new era of assistance immunotherapy. At the same time, it also obtained the recommendation of authoritative guidelines such as the National Comprehensive Cancer Network (NCCN), the American Clinical Oncology Society (ASCO), and the Chinese Clinical Oncology Society (CSCO). "
After the main endpoint of the IMPOWER010 research reaches the DFS, OS data as a key secondary end point is highly anticipated by clinic workers. The data released by WCLC is the first OS mid -term analysis results carried out by the IMPOWER010 research plan. The median follow -up time of the patient was 45.3 months (as of April 18, 2022).
Professor Zhou Cun interpreted that in Phase II-IIIA patients with PD-L1 TC ≥ 1%, the Adidizumab Anti-Aid therapy group has a significant OS benefit trend (HR = 0.71,95%CI: 0.49-1.03 ), The 3 -year OS rate of patients with the treatment group is 82.1%, and the 5 -year OS rate is 76.8%. The survival curve diagram has also showed a relatively obvious separation trend from the control group (Figure 1).
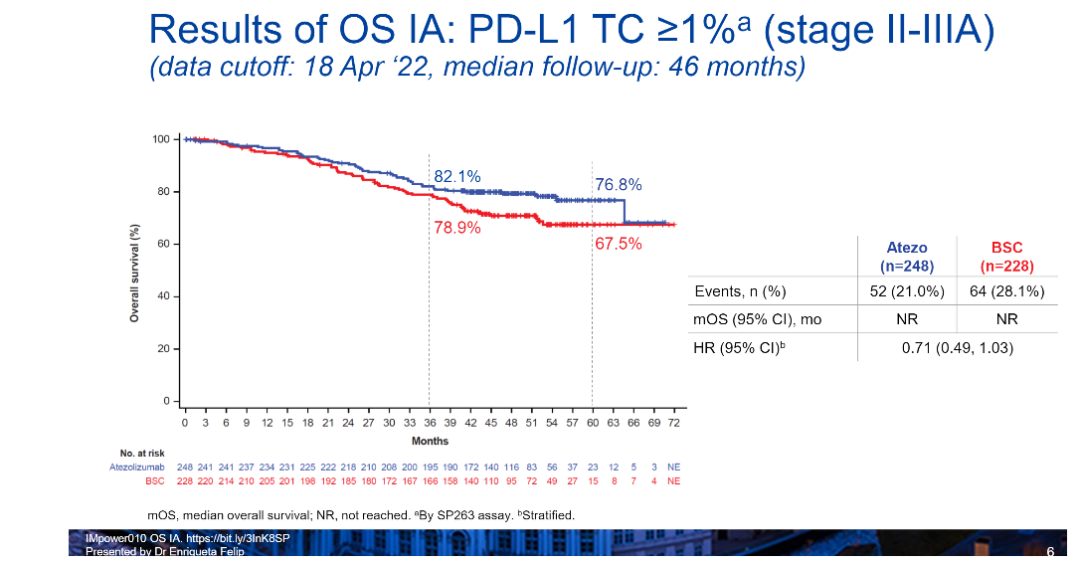
Figure 1. OS data of Phase II-IIIA patients withpd-L1TC ≥ 1%
It is worth noting that among PD-L1 TC ≥ 50%of patients with Phase II-IIIA, the OS benefits of Ayidzumab treatment are more prominent (HR = 0.43,95%CI: 0.24-0.78), treatment The 3 -year OS rate of patients with groups was 89.1%, and the 5 -year OS rate was 84.8%. The 5 -year OS rate increased by nearly 20%compared with the control group (Figure 2).
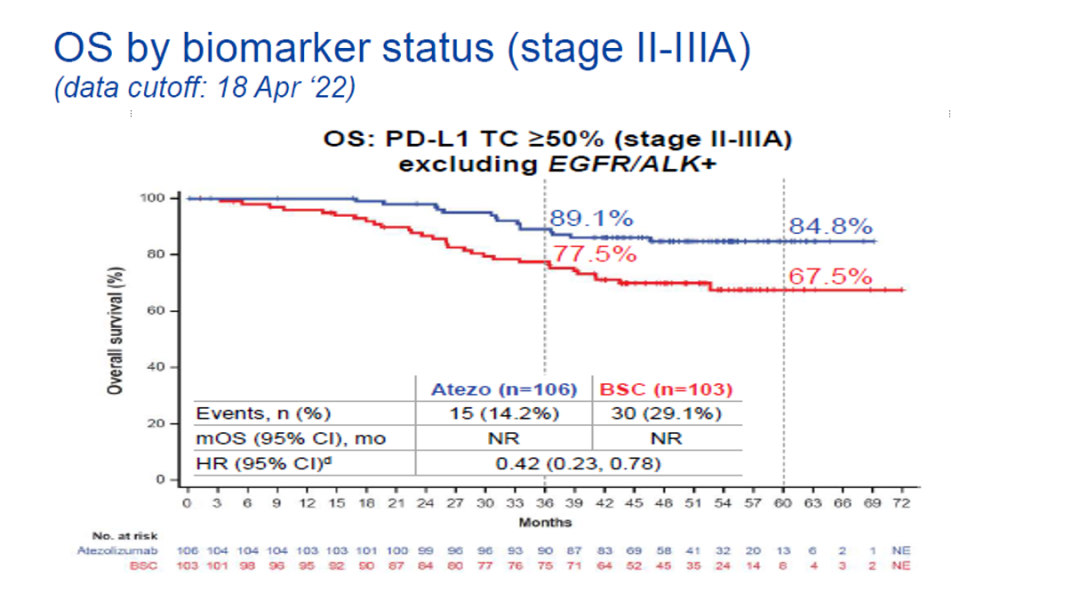
Figure 2.PD-L1 TC ≥ 50%of Phase II-IIIA patient OS data
In addition, the study also further analyzed the Asian group of OS data of PD-L1 TC ≥ 1%of the OS data OS data. The results show that among the key sub -groups such as different diseases and lymph nodes violations, Ayidarzab can observe the obvious trend of benefit, and further verify the efficacy of Ayiduzumab as the assisted treatment of NSCLC surgery. (image 3).
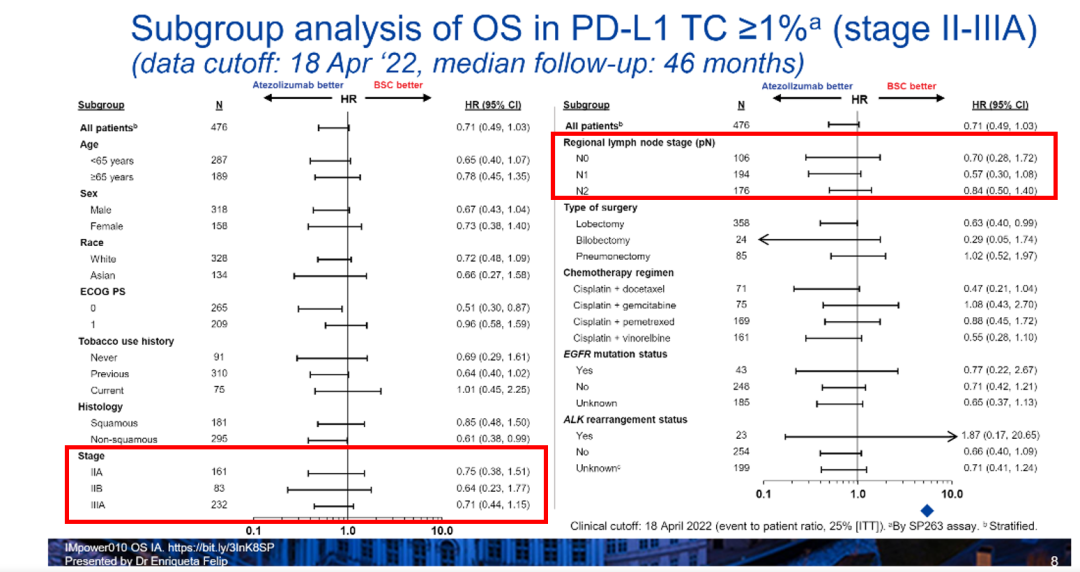
Figure 3. Phase II-IIIA patients in Phase II-IIIA in Phase II-IIIA in Phase IIII in Phase IIIA
Professor Zhou Cun said, "The previous platinum -containing dual -drug auxiliary chemotherapy can only increase the patient's 5 -year OS rate by 5%[2], and the auxiliary targeted therapy has not seen OS benefits. Anti-auxiliary treatment can increase the 5-year OS rate of 10%, especially for high-expression patients to increase by nearly 20%, which is very great achievement. These data also suggest that we are the II-IIIIA of PD-L1 TC ≥ 1% Patients should conduct Ayidarzumab auxiliary immunotherapy, especially patients with PD-L1 TC ≥ 50%. The level of chemotherapy really allows patients to have the possibility of cure. "
Professor Zhou Cun also said that the IMPOWER010 research data announced this time successfully proved that DFS benefits were transformed into long -term OS benefits, and further confirmed that Ayidarzumab Aipuradimal Aid therapy was currently a new auxiliary therapy standard scheme. Essence
Ayidzumab assisted immunotherapy is safe and reliable to protect early lung cancer patients
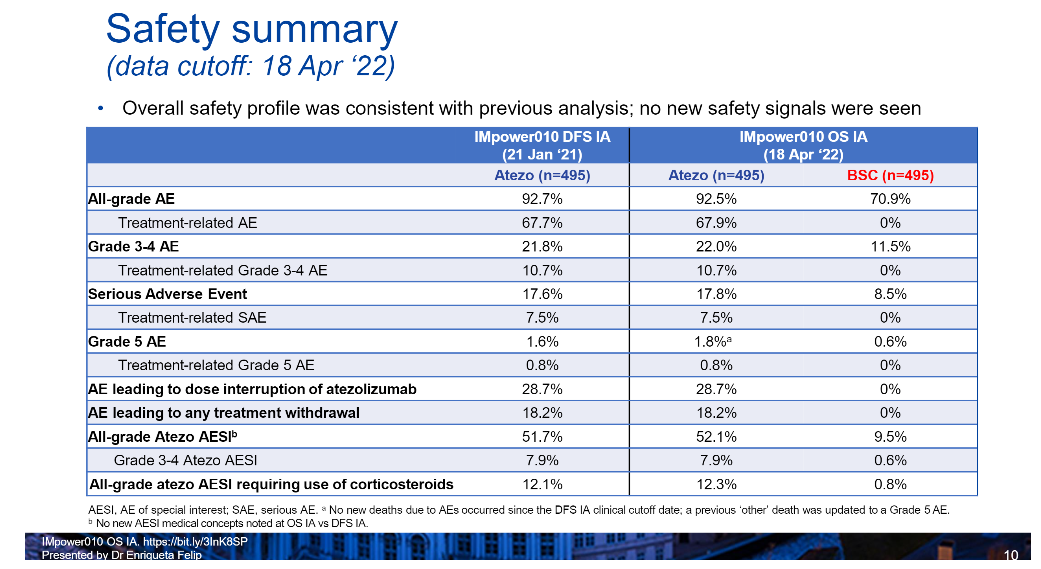
Due to the relatively longer medication cycle of NSCLC after surgery, such as in Impower010 research design, after the patient completes surgery and auxiliary chemotherapy (1-4 cycles), the long time of Aidarzumizumi is 1 year for treatment related to treatment. The problem of safety and patient tolerance is also concerned about the concerns of surgery, internal medicine clinical workers and patients. The safety data released before shows that the safety of Ayidzumabs has good safety. The 3-4 level of treatment related adverse events (Traes), the treatment of serious adverse events (SAES) and adverse events are reduced/stopped/stop The occurrence rate of medicine is low, and patients are tolerant of treatment.
Professor Zhou Cun said that the security data released by WCLC is the result of the first main DFS analysis after the first time the results of the main DFS analysis. The relevant data is basically consistent with the data reported before: the study has not found a new security signal, Adidili Lili is not found, and Adi Lilies The incidence of level 3-4 TRAES, Saes, and Drug/Reduction of the Pearl Mipido Aid therapy did not change significantly due to the extension of the follow-up period (see Table 1).
Table 1.IMPOWER010 Study and updated security data
In this regard, Professor Zhou Cun also pointed out that "different ICI toxicity is different. The safety of PD-L1 inhibitors is better than that of PD-1 inhibitors. The highest security [3], in addition, we also observed from the patients who studied the group that the incidence of level 3-4 Traes is quite low, and most patients are tolerated. It has been approved in China to treat small cell lung cancer (SCLC), PD-L1 high expression transfer NSCLC, and metastatic non-scale NSCLC, which further confirms the efficacy and safety of Ayidizumab. Domestic clinical clinical clinicals Workers have rich experience in the application of Ayidi Midurate. I believe that the application of Ayiduzumab aid therapy will also be safe and efficient. "
Explore and optimize biomarkers,
Make auxiliary immunotherapy more accurate
Through PD-L1 expression level, circulatory tumor DNA (CTDNA) and other biomarkers, refine the selection of patients and achieve more accurate and individualized treatment. Carried out relevant analysis.
Professor Zhou Cun introduced that PD-L1 expression level is currently the most important symbol for immunotherapy for advanced NSCLC. It can guide the selection of treatment plans and predict the clinical benefits of patients. The IMPOWER 010 study further confirmed that PD-L1 expression level in early NSCLC was also an important biomarker. IMPOWER 010 research uses two PD-L1 detection in SP142 and SP263 in design, of which SP263 detection is relatively wide in China. Analysis of research data shows that the expression level of PD-L1 detected by the two platforms has a good correlation with the curative effect of assisted immunotherapy, which plays a role in verifying each other [4]. The OS analysis data released by WCLC also further supports the use of auxiliary immunotherapy for patients with positive (TC ≥ 1%) for PD-L1. Therefore, clinicians should strengthen the attention of the PD-L1 testing of NSCLC patients, accurately choose the advantageous people who can benefit from the auxiliary immunotherapy, and bring greater benefits to patients.
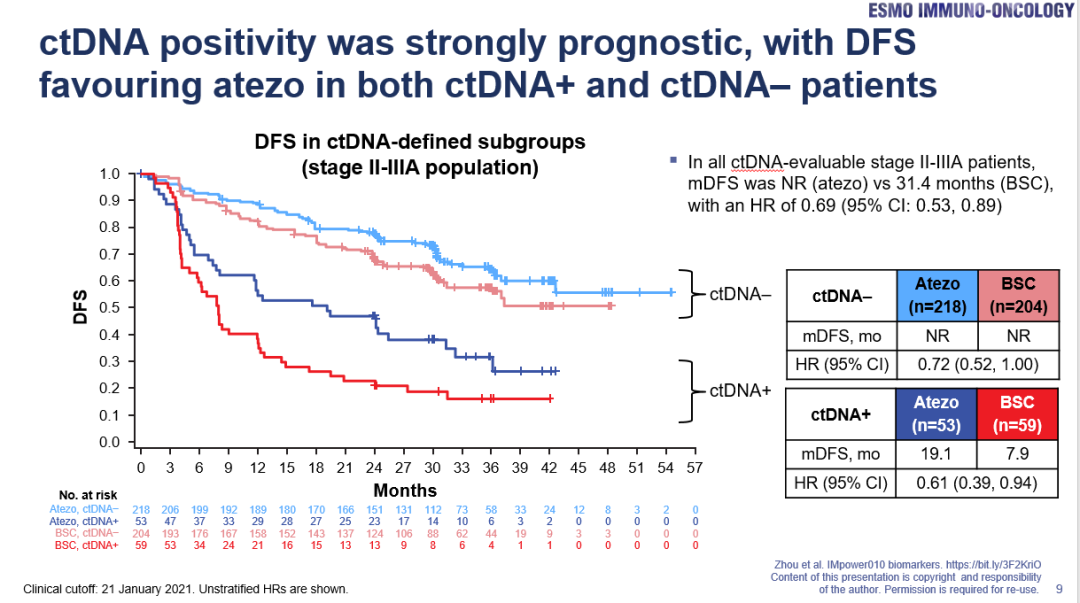
In terms of other biomarkers, Professor Zhou Cun believes that from the existing data from IMPOWER010, the two major genetic positive patients with EGFR/ALK are not recommended for the first choice of assist immunotherapy, and corresponding auxiliary targeted therapy can be considered; and the corresponding auxiliary targeted therapy can be considered; and the corresponding auxiliary targeted therapy can The analysis published last year in the ESMO-IO report also showed that patients' postoperative detection CTDNA positive is the adverse prognosis of DFS, but whether the CTDNA positive is detected, the Ayiduzumab assistive immunotherapy, PD-L1 TC ≥ 1 %Of the II-IIIA patients have DFS benefits (Figure 4), suggesting that CTDNA has important value in predicting the prediction of adverse prognosis, so it is not advisable to use CTDNA for efficiency prediction.
Figure 4. Regardless of whether the CTDNA test after the patient is positive, the Ayidarzab Anti -Aid therapy has DFS benefits
The IMPOWER010 research results of the IMPOWER010 updated in WCLC are also a milestone in the perioperative immunotherapy. It also provides inspiration and guidance for subsequent clinical exploration. Professor Zhou Cun believes that "IMPOWER010 studies confirmed the value of immune single drug assisted therapy and opened a new era of assisted immunotherapy. Now the new assisted therapy for immunochemical+chemotherapy has also been established, but there are still many explorers on the treatment of perprore surgery. For example, the exploration of the best treatment mode of perioperative surgery period has a number of clinical studies that combined with new assisted immunity and auxiliary immune immune like Impower030. The results will be announced in succession next year. The best cycle of auxiliary/auxiliary therapy, as well as the duration after surgery interval, is a direction worth exploring. "
In addition, PD-1/L1 inhibitors and other immunotherapy drugs, such as the approved CTLA-4 inhibitors, are used for clinical research on Tigit, NKG2A, CD73 and other targets. It is an important direction worthy of attention. However, Professor Zhou Cun said that the clinical research of early NSCLC often used a long time and a large amount of samples. Therefore, the prospects of immune+immunohistochemical treatment need to be further observed. Expert Introduction
Professor Zhou Cun
Shanghai Lung Department, affiliated to Tongji University

Executive Member of the China Clinical Oncology Society
Chairman of the Chinese Medical Promotion Association's chest tumor branch
IASLC BOD
Chairman of the Non -Small Cell Special Committee of the China Clinical Oncology Society
Standing Committee Member of the China Anti -Cancer Association Lung Cancer Professional Committee
Deputy Chairman of the Shanghai Anti -Cancer Association
Chairman of the Shanghai Anti -Cancer Association Pulmonary Cancer Molecular targeted and immunotherapy
Deputy Chairman of the Professional Committee of Cancer Association of the China Anti -Cancer Association
Standing Committee Member of the Chinese Medical Association Tumor Branch
Vice President of the Tumor Branch of the Shanghai Medical Association
Deputy Chairman of the Tumor Branch of the Shanghai Medical Association
Director of the Institute of Cancer Institute of Tongji University Medical College
Shanghai leader
references:
[1]Felip E,Altorki N,Zhou C,et al.Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer(IMpower010):A randomised,multicentre,open-label,phase 3 trial[ J]. The Lancet, 2021,398 (10308): 1344-1357.
[2]Group N M C.Adjuvant chemotherapy,with or without postoperative radiotherapy,in operable non-small-cell lung cancer:two meta-analyses of individual patient data[J].The Lancet,2010,375(9722):1267- 1277.
[3] Wang Y, zhou s, yang f, etreatment-related adverse events of pd-l1 inhibitors in clinical trials: a systematic review and meta -nalysis [j] .jama onCology, 2019,9. (7): 1008-1019.
[4]Zhou C,Thakur M D,Srivastava M K,et al.2O IMpower010:Biomarkers of disease-free survival(DFS)in a phase III study of atezolizumab(atezo)vs best supportive care(BSC)after adjuvant chemotherapy in stage IB -IIIA NSCLC [J] .annals of oncology, 2021,32 (s7): s1374.
Source: Medical Community
Responsible editor: Zheng Huaju
School pair: Zang Hengjia
Hot text recommendation
- END -
Provincial Consumer Protection Commission issued compliance guidelines to standardize e -commerce ultra -long pre -sale
I obviously bought spring clothes, but in summer, I have not received it. I have waited for two months to wait for the goods to be purchased. From the spot to 7 days to pre -sale, then 15 days, 30
Facing high temperature burning roasting and guaranteeing power, Baoshan "Electricity Man" tens of thousands of steps to send "cool" to 10,000 house

In the past few days, Shencheng has issued high -temperature red and orange warnin...