Can you drink heavy water participating in nuclear reactions?Have you said sweet?
Author:Beijing Science Center Time:2022.08.05
Review expert: Gan Qiang
Lecturer of Beijing Institute of Technology, Doctor of Applied Chemistry
What is water? From the beginning of junior high school contact chemistry, we have firmly remembered that the water is H2O, so this seems to be a stupid problem, but chemists don't think so.
Everyone knows this incident, but if there is an ice cubes above 3.8 ° C, you can melt. Do you believe it? It does not require any special pressure or other environmental conditions, nor is it mixed with any other substances, or pure, authentic water, but it is a bit different. It is called heavy water.

The left side is heavy water ice cubes, and the right side is ordinary ice cubes
Where is heavy water "heavy"?
In simple terms, heavy water is only density than ordinary water (we call "light water"). Micro, ordinary water and heavy water molecules are composed of 2 hydrogen atoms and 1 oxygen atoms. The difference is that the hydrogen atomic symbol in ordinary water is H, which is called "hydrogen" (or "氕" (or "氕" (or "氕 氕" (or "氕 氕" (or "氕 氕" (or "氕 氕" (or "氕 氕" (or "氕 氕" (or "氕 氕" (or "氕 氕" (or "氕 氕" (or "氕 氕" (or "氕 氕" "), And the hydrogen atomic symbol in heavy water is D, which is called" heavy hydrogen "(or" 氘 ").
What is the difference between H and D? In fact, the atomic nucleus of the ordinary hydrogen atom H contains only one proton, and a neutron is added in the atomic nucleus of the heaviel hydrogen atom D. In addition, the third type of hydrogen atom is T, which is called "ultra -weight hydrogen" (or "氚"), and it is also 2 neutron among the nucleus.
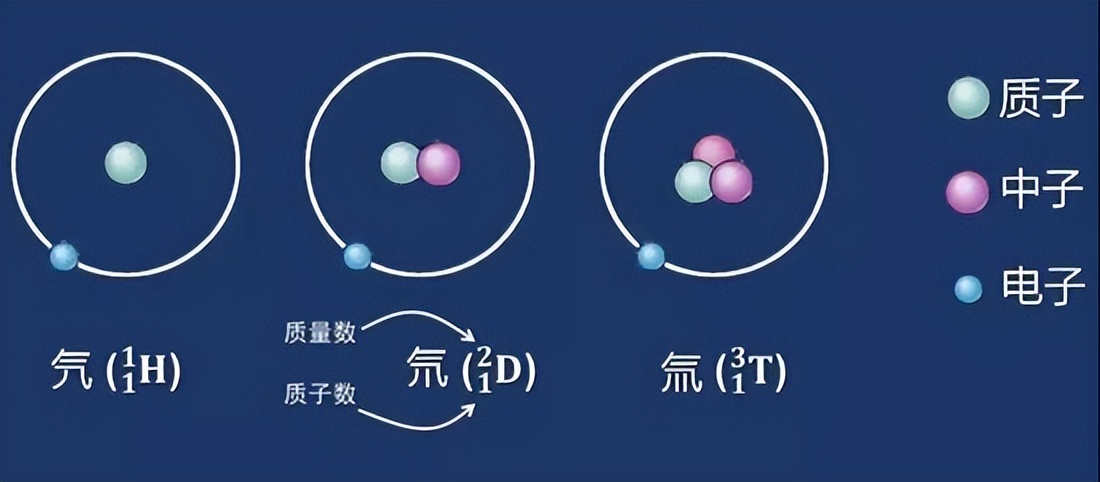
From the appearance point of view, there is no difference between heavy water and ordinary water. They are colorless liquids, and because they are substances for the same element, their chemical properties are very close, but there is no difference at all. No one believes.
In nature, the distribution of heavy water is very uneven, and the content of heavy water in snow, rain, and water in the surface water is very small. There are about 7.5 kg of heavy water per 50 tons of ordinary water. Therefore, in order to obtain heavy water, the method of electrolytic water is generally used. Because heavy water will not be electrolytic, the concentration of heavy water in the electrolyte will become higher and higher, and eventually get more pure heavy water. However, the heavy water prepared in this way will consume a lot of electricity, and 1 kg of heavy water will be 3 times more than the power required for melting 1 tons of aluminum.

So why do people still electile water to prepare heavy water? This is because in modern atomic reactors, the rate of heavy water participation in chemical reactions is slower than light water. It is the best neutron reducer so far. Make chain reactions sustainable.
Evergreen is toxic?
As soon as the heavy water begins to enter the people's vision, it is associated with the nuclear reactor. Although we already know that it is not dangerous now, many people's first impression of it was extremely dangerous at that time.
However, the curiosity of scientists is endless, and soon after it was found, someone drank heavy water.
This person is George Charles de Hevesey. The name may be strange to most people, but if someone has heard the story of dissolving the gold medal of the Nobel Prize with Wang Shui, you can also see this one from this. The temperament of scientists. But that's another story, which has nothing to do with heavy water today. To be home, we first consider its toxicity for drinking heavy water.

Although heavy water is not radioactive, it is not toxic substances, but creatures only need ordinary water. This is as if nitrogen in the air is non -toxic, but inhaling high concentrations of nitrogen will be hypoxic and suffocating. If you only drink heavy water, your physical function will definitely have problems.
It has been found in the mouse experiment that heavy water can inhibit the division of cells (60%of the death concentration of heavy water), resulting in tissue that needs to be rapidly metabolized due to large cell deaths. There are problems. The good news may be that the rapid growth of cancer cells will also slow down, but the degree of slowing is not much helpful for the treatment of cancer.

Plants will gradually wither in a high -concentration heavy water environment | Thesis
It can be clear that the heavy water currently bought through regular channels will basically mark a safety warning on the bottle body tag, or "only for experiments" and other reminders. It is not recommended that anyone try to drink heavy water.

Is heavy water sweet?
But theory is theory. Drinking a little problem is not big, so there are countless people who have tried the taste of heavy water. As for the reason for making so many people "chasing hot spots", it is a bit strange -Is heavy water sweet?

Source | Popular Science Report Election
Why is water sweet? According to a curious blogger's plan, he summoned and set up simple blind test experiments. The subject will taste different types of water in turn, three drops each time. In order to exclude irrelevant factors, such as the difference between the senses brought by the weight, he also bought a heavy money to buy the same oxygen hydrogenation (also known as oxygen 18 water). As a result very fast.

Source 丨 Thunderf00T
In another taste test experiment, 22 of the 28 participants can accurately distinguish heavy water. According to the feedback from the participants, the sweetness of pure water is the most significant, but the overall taste is light, with an average sweetness of 3.3 ± ± 0.4.
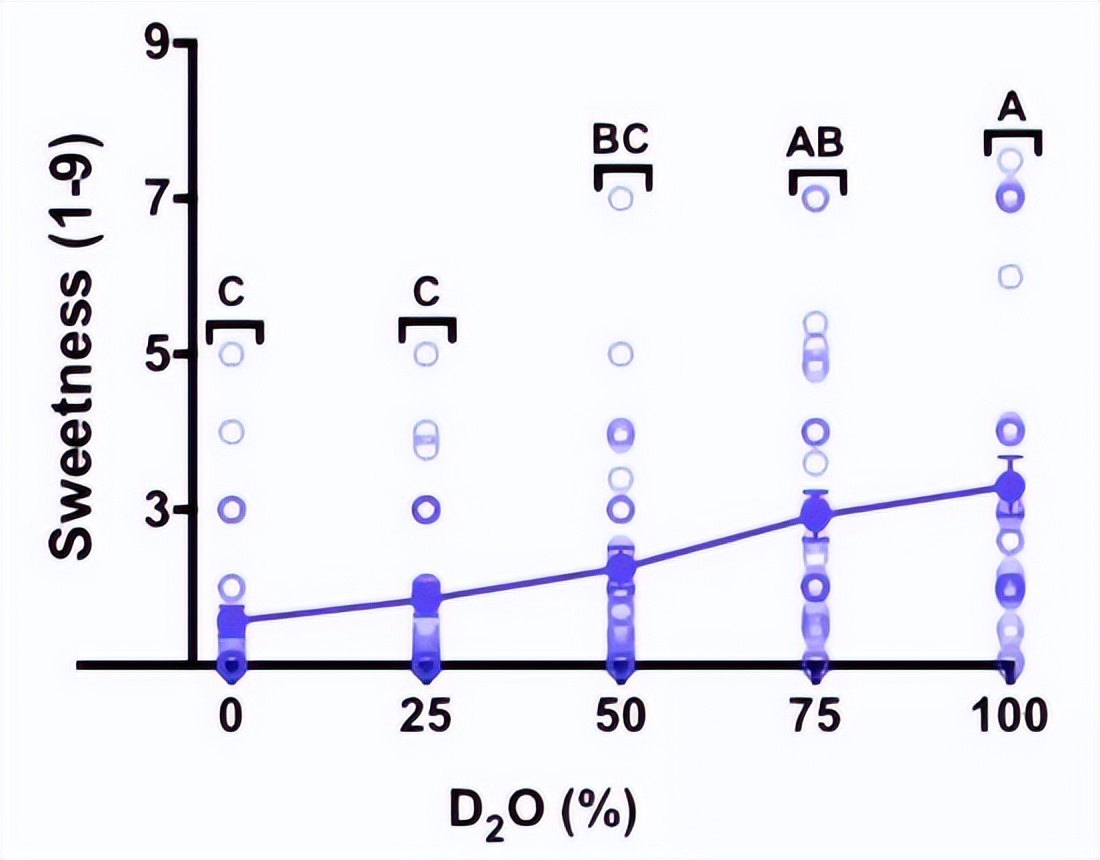
Sweet sense perception (1 without sensation, 3 mild, 5 medium, 7 very, 9 齁 sweet)
In order to further study the cause of heavy water to produce sweetness, the second experimental team also conducted mouse experiments, but found that mice have a strong preference for sucrose water, but they have no sense of heavy water, and even observed that the mice showed showing that the mice showed that it showed that the mice showed that it showed that the mice showed that it showed that the mice showed that it showed that the mice showed that it showed that the mice showed that it showed that the mice showed that it showed that the mice showed that it showed that the mice showed that it showed that the mice showed that it showed that the mice showed that the mice showed that the mice showed that the mice showed that the mice showed that the mice showed that it showed that the mice showed that it showed that the mice showed that it showed that the mice showed that it showed that the mice showed that it showed that the mice showed that it showed that the mice showed itAversion to heavy water.Source | Paper "A Study of Taste and SMELL of Heavy Water (99.8%) in Rats"
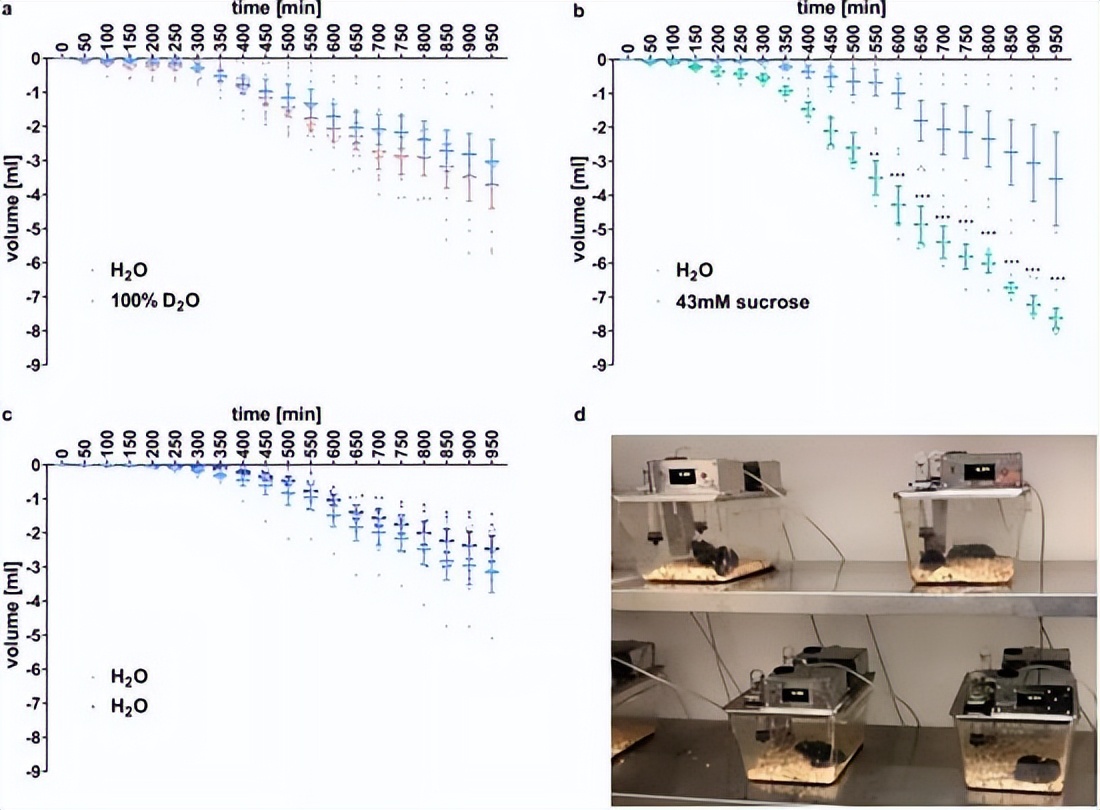
This brings a guess: The reason why heavy water can taste sweet may come from human beings, and rodent creatures do not have sweet taste receptors.
Source | Carmelo Tempra / IOCB PRAGUE

Further experiments confirmed that TAS1R2 / TAS1R3 receptor is the ultimate cause of the sweetness of human beings to taste heavy water, and subsequent receptor inhibitors experiments also prove this point.It has to be further studied and discovered.
In short, there is a useless little knowledge here: when you have two glasses in front of you, you can judge which cup is heavy water, but cherish life, please stay away from heavy water!
- END -
The latest progress of the Shenzhen -Middle Channel!

On July 22, the Guangdong Provincial Transportation Group released the news that t...
If you are well, I will be relieved

Nine, flying snow, cold. Small snow particles rammed the window pupa, and the soun...