Nejm: Repeating lung cancer use Nawuli Ulitab neo -assisted treatment can increase clinical income
Author:Cancer Channel of the Medical Time:2022.07.21
*For medical professionals for reading reference

New assisted immunotherapy is progressing!
In the May 2022 issue of NEJM, a study shows that [1] can remove the new auxiliary scheme of lung cancer with Nawuli Mipida, which can increase clinical revenue, including extending the incident -free survival period (EFS), increasing the pathological relief rate (original originality Tumor and sample lymph nodes remain 0%), and will not increase adverse reactions, nor will it affect subsequent surgical treatment.

About 20%to 25%of patients with non-small cell lung cancer (NSCLC) can be surgical resection, but 30%to 55%of patients will relapse after surgery and eventually die from lung cancer recurrence [2-3]. Although the auxiliary therapy of NSCLC has made progress, even if the patient's lung cancer can take surgical resection for local treatment, systemic treatment is still required to reduce postoperative recurrence and improve clinical benefits. Nawuli Ulitab is a PD-1 antibody that can restore the anti-tumor ability of T cells, directly or indirectly activate the immune system, and enhance the patient's antitumor immunity.
Prior to this, the plan based on the Nawuli Mippitive has shown the survival benefits used to remove patients with NSCLC.
In 2020, a Study on a Phase 2 Study on the Open Tag Multi -Center single -arm 2 stages of Lancet Oncol also confirmed that surgical resection NSCLC patients have certain benefits to the use of Nawli Ulum Mipida for combined chemotherapy [4]. The study incorporated 46 patients with removable NSCLC patients. Patients adopted 3 new auxiliary chemotherapy before surgery. Wuli Yumab (360 mg), continued to use Nawuliyabu to maintain treatment for 1 year after surgery. The research was followed up for 24 months, and the results showed that 41 patients eventually removed the tumor and did not progress 35 cases. There was no progressive survival (PFS) rate of 77.1%(95%CI 59.9%~ 87.7%) in 24 months. There was no case of death during the neo -assisted chemotherapy period, and none of them affected subsequent surgical treatment.

NEJM published a Nawuli Mipida combined with platinum dual-drug chemotherapy treatment of NSCLC open label 3 clinical research (Checkmate-816). This study is currently the first III clinical trial based on immunotherapy based on immunotherapy in patients with positive results in patients with positive results in patients with resection NSCLC patients. In CheckMate-816, compared with individual chemotherapy, the new assisted chemotherapy scheme based on Nawuli Mipida is used to remove patients with NSCLC. The long -term clinical ending of patients with NSCLC will not hinder the feasibility of surgery or increase the incidence of adverse events.
Arrangement standard
The patients incorporated in the study belong to the IB (≥4 cm) stage, and the ECOG PS score is 0 or 1 point (the total score is 5 points, the higher the score, the higher the degree of disability), and the diagnosed NSCLC that has been diagnosed with anti -cancer treatment. patient. Evaluate the expression of PD-L1 before the treatment of patients for treatment. Excluding patients with known ALK or EGFR mutations.
Research methods
A total of 773 patients were recruited from March 2017 to November 2019, of which 505 patients received random chemotherapy, including 179 cases of 179 cases of platinum dual drug chemotherapy receiving combined Nawuli Mipida (360 mg) complacent dual drug chemotherapy chemotherapy (360 mg) Patients (incorporated into the combined Nawuli Yibuura Therapy group) and 179 patients with pure platinum chemotherapy (incorporated into the simple platinum chemotherapy group), chemotherapy treatment was once every 3 weeks, with a total of 3 courses. In the end, 176 patients in each group were treated.
Research result
The median survival period of the United Na Wuli -Bimaguyotherapy Group is 31.6 months (95%CI, 30.2 to not reached), and the median survival period of the platinum dual -based dual -drug chemotherapy group is 20.8 months (95%CI, 14.0 14.0 ~ 26.7) (HR: 0.63; 97.38%CI, 0.43 ~ 0.91; P = 0.005). See below. The 1 -year EFS rate of the 1 -year EFS rate of the combination of Nawuliyu's anti -chemotherapy group was 76.1%, and the pure platinum -containing dual -drug chemotherapy group was 63.4%; the 2 -year EFS rate was 63.8%and 45.3%, respectively.
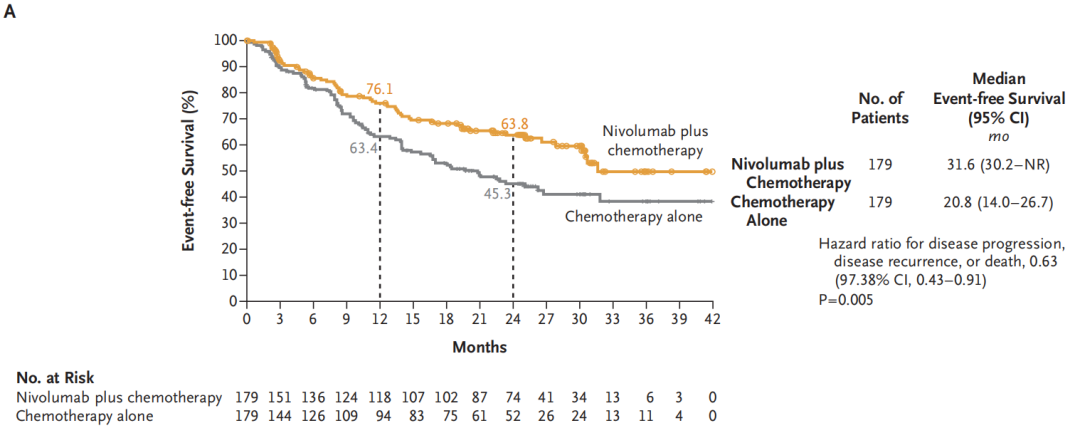

Figure A shows the EFS of patients who receive random groups at the same time, and Figure B shows the EFS of the sub -group of patients with a pre -specified patient.
The proportion of subsequent surgery for the two groups of patients was 83.2%of the combination of 83.2%of the combination of Nawuli Mipide and 75.4%of the pure platinum dual pharmaceutical chemotherapy; 15.6%and 20.7%of patients were canceled. The causes of cancellation include the progress of the disease (6.7%and 9.5%), the adverse events (1.1%and 0.6%), and the other [7.8%and 10.6%(including the patient's rejection, non -removal and lung function). The proportion of patients with delayed surgery in the two treatment groups is similar, and the differences are not statistically significant. From the figure, compared with the pure platinum -containing dual drug chemotherapy group, the duration in the operation of the combined Na Wuli Uyoubei Anti -chemotherapy Group is shorter, minimally invasiveness is more common, and lung resection is rare.
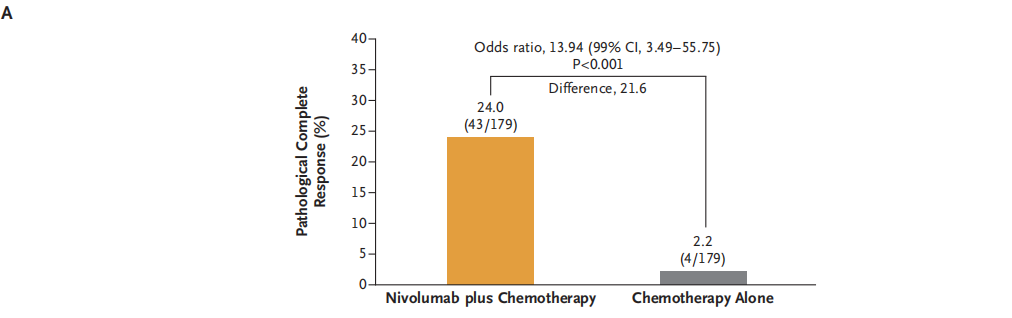
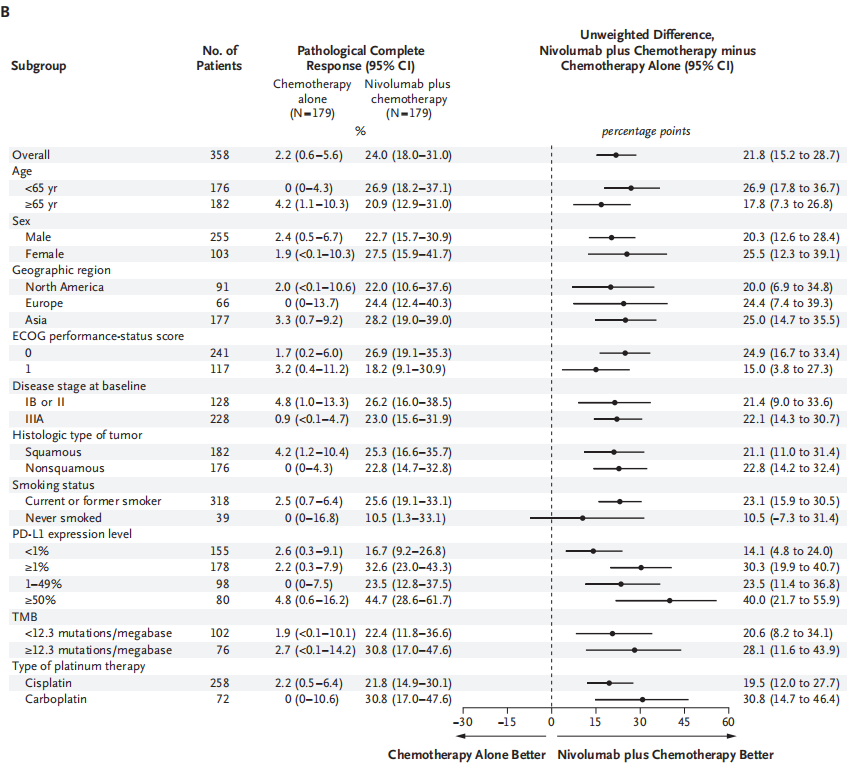
The complete relief rate of combined with Nawu Liyab's anti -chemotherapy is 24.0%(95%CI, 18.0%~ 31.0%), and 2.2%(95%CI, 0.6%~ 5.6%) of pure platinum dual pharmaceutical chemotherapy (95%CI, 0.6%~ 5.6%) OR value is 13.94; 99%CI, 3.49%~ 55.75%; P <0.001). The pathological relief rate of all key sub-groups of the combined Nawuli anti-chemotherapy is high, including the sub-group based on the staging of the disease, the tumor PD-L1 expression level, and the organizational type. Figure A shows the comparison of the pathology of the two main analysis of the two groups. Figure B shows the comparison of the pathological alleviating of pathology in the specified sub -group.
The incidence of non -performing incidents in level 3 or level 4 of the combination of Nawu Liyouzumabi was 33.5%, compared with 36.9%of the pure platinum dual pharmaceutical chemotherapy, and the difference was not statistically significant. The percentage of delay surgery was 3.4%due to adverse events in combination with Nawu Liyu, and 5.1%of the platinum dual -containing dual drug chemotherapy group; 1.1%and 0.6%of the cancellation surgery, respectively. Two patients in the combination of Nawuli Mipidicotherapy reported that there were no adverse events related to level 5 surgery, but the researchers believed that these adverse events had nothing to do with test drugs (one example of pulmonary embolism and aorta rupture).
The research results show that the use of NSCLC patients with NSCLC patients with Platinum Double Pharmaceutical Chemotherapy adopt no event. The incidence of adverse events in level 4 treatment does not affect subsequent surgical solutions.
references:
[1] Forde Patrick M, Spicer Jonathan, Lu Shun, et al.NEOADJUVANT Nivolum Chemotherapy in Resectable Lung Cancer. [J] .n Engl J Med, 2022.https: //www.nejm.org/do /do /do /do /do /do /do /do /do /do /do /do /do /do /do /do /do /do/www.nejm. 10.1056/nejmoa2202170
[2] Uramoto H, Tanaka F.Recurrence after Surgery in Patients with NSCLC.TRANSL LUNG CANCER Res, 2014,3: 242-249.
[3] Taylor MD, Nagji as, Bhamidipati CM, et al.Tumor recurrence after resection for non-Small Cell LUNG Cancer.ann Thorac Surg, 2012,93: 1813-1820.
[4]Mariano Provencio,Ernest Nadal,Amelia Insa,et al.Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer(NADIM):an open-label,multicentre,single-arm,phase 2 trial.The Lancet Oncology .SePtember 24,2020.
Frontier of the tumor you want
Please pay attention to the doctor station 注
1. Scan the QR code below
2. Click to download the app

3. Open the doctor station app and click on the upper right corner
4. Find the tumor in the channel
And follow
Download the doctor's station app and subscribe anytime, anywhere ~
The first release of this article: the medical world tumor channel

Author of this article: Beauty Egg
Review of this article: Xu Weiran
Editor in charge: Sweet
- END -
【Mountain Camellia】 Shake the fan to send cool
The electric fan replaces the fan, the air conditioner replaces the electric fan, showing the progress of the times. However, recently, I found that my father picked up the old -fashioned big pan fan
@, The Municipal Consumers Association reminds "6 · 18" online shopping!
Dahe Daily · Henan Video Reporter Liu Guangchao Correspondent Guo YongruiToday is June 18, and major e -commerce platforms have launched various formal discount promotional activities to attract co