CAR-T further!New progress of aggressive lymphoma treatment
Author:Cancer Channel of the Medical Time:2022.07.21
*For medical professionals for reading reference
Give you the most cutting-edge CAR-T information
From June 9-17, 2022, the 27th European Hematology Association (EHA) Annual Conference was held in Vienna, Austria through the form of online+offline group conferences. As the largest international conference in the field of blood in Europe, it attracts professionals from global professionals to share cutting -edge breakthroughs every year. Xiaobian's latest research abstracts for invasive lymphoma CAR-T cell therapy, let's see what wonderful highlights!

Scan the two -dimensional code above, the blood information is fast, one step
1. CAR-T cell therapy therapy for acute toxicity of non-Hodgkin B-cell lymphoma: Real World Studies based on DESCAR-T registration
Title: Car T-Cells Associated Acute Toxicity in B-Cell Non-Hodgkin Lymphoma: Real-WORLD Study from the Descar-Tregistry
Summary number: S210
In CAR-T cell therapy, cytokine storm (CRS) and immune effects cell-related neurotoxic syndrome (icans) are common immune-related toxic reactions. Its clinical manifestations can be very serious and may endanger life. This study will analyze real-world data, find out high-risk factors that lead to a non-performing reaction of CAR-T, and guide clinical practice.
The study collected the recurrence/refractory invasion B-cell lymphoma data for CAR-T in the DESCAR-T registration at the French DESCAR-T, and classified CRS/ICAN degree (ASTCT grading). Evaluate the score and simplified Easix score.
Studies were included in the data of 705 patients, of which 74 patients were ECOG PS≥2 before the lymphocyte was cleared. The median follow -up time is 12 months:
587 (83.3%) patients had a CRS, of which 62 bits occurred 3 and above CRS. The median time from the return to the occurrence of CRS is 2 days (range: 0-34 days), and the median fade time of CRS is 6 days (range 1-30 days).
The total number of cases of ICANS is 289 (41%), of which 3 and above 78 (27%). The median time from the return to the occurrence of ICANS is 6 days (range: 0-379 days), and the median fading time of ICANS is 6 days (range 1-100 days). Most patients who have icans will experience CRS first.
Todonab is the most commonly used drug (411 cases, 68%) for the treatment of CAR-T toxic reactions, and 272 patients received steroid hormone therapy.
Patients with 38%occurred CRS, 53%occurred 2 CRS, and 65%of CRS occurred.
Researchers conducted multi -factor analysis and found that high -risk factors that caused CRS and icans include:
The elevation of AXI-CEL and LDH can predict CRS. Tumor size is 5cm, age <65, and higher Seasix scores will increase the risk of level 3 CRS.
AXI-CEL, ECOG ≥ 2, age ≥65 years and high Seasix scores can predict iCANS. Axi-Cel, ECOG ≥ 2, higher Seasix rating increases the risk of ≥ 3 ≥ 3 ≥ 3.
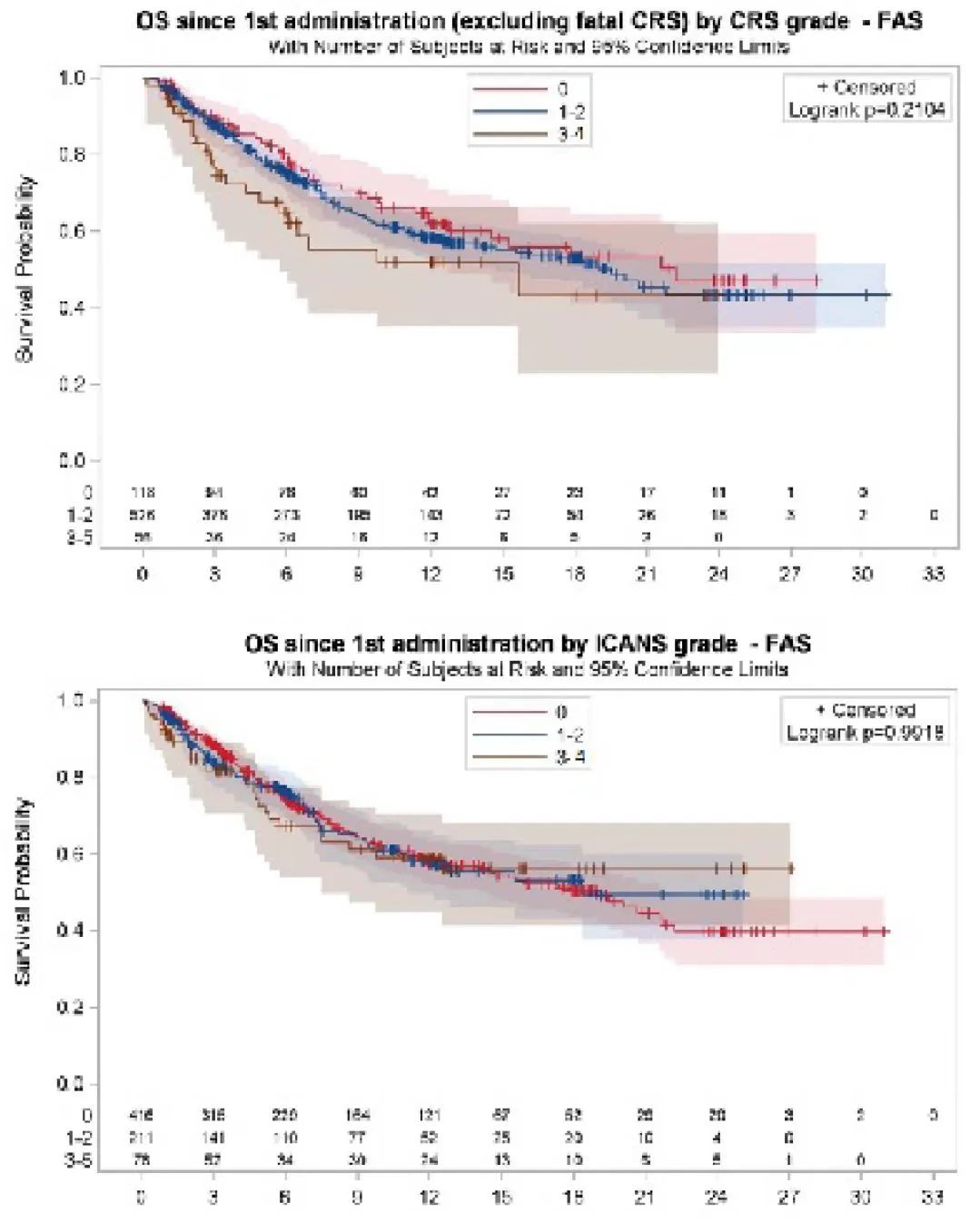
In addition, the survival analysis of patients after CRS and ICANS found:
≥ 3 adverse incidents will not affect the patient's OS and PFS.
The cumulative dosage and duration of the niphriposumerical and steroid hormones will not cause negative effects on PFS and OS.
2. ZUMA-7: Revelation/Difficulty B cell lymphoma uses AXI-CEL to compare the real world data for standard therapy
标题:CLINICAL AND PATIENT-REPORTED OUTCOMES IN A PHASE 3 STUDY OF AXICABTAGENE CILOLEUCEL(AXI-CEL) VS STANDARD-OF-CARE IN ELDERLY PATIENTS WITH RELAPSED/REFRACTORY LARGE B-CELL LYMPHOMA (ZUMA-7)
Summary number: S211
The prognosis of recurrence/refractory large B -cell lymphoma (R/R LBCL) is usually poor, the probability of adverse incidents increases, and the second -line standard treatment (SOC) tolerance is poor. In addition, second -tier SOC often leads to a decline in quality of life in patients.
The global and III-stage random control research Zuma-7 studies have shown that AXI-Cel can significantly improve the patient's non-incident survival period (EFS) compared to the second-line SOC. The EHA conference reported the analysis results of the Zuma-7 Asian group.
Patients with R/R LBCL patients with 0-1 and first-line treatment time ≤ 12 months, and randomly allocated to the AXI-CEL or SOC group at a ratio of 1: 1, partially relieved (PR) or patients with complete relief (CR) continues to continue Carry autologous stem cell transplantation. At the same time, at the baseline, D50, D100, D150, 9 months, and every 3 months, the patient's quality of life is evaluated, until 24 months or safety or survival events. As of March 18, 2021, 51 patients received AXI-CEL treatment and 58 patients who were treated with SOC treatment could be evaluated. The median age is 70 years (65-80 years old). Compared with the SOC treatment group, patients with AXI-CEL treatment group have more high-risk factors, such as the second-tier age adjustment international prognosis index of 2-3 (31% vs 53%) and LDH elevated (41% vs 61%). Survival analysis shows that the EFS of the AXI-CEL treatment group is higher than that of the SOC treatment group (HR = 0.276, P <0.0001), and has a higher complete relief (CR) rate (75% vs 33%).
Level 3 and above treatment related adverse events (Teaes) in the AXI-CEL treatment group and SOC therapy groups were 94%and 82%, respectively. It is worth noting that the number of cases of level 5 of the AXI-CEL treatment group is 0, while the number of cases in the SOC therapy group is 1.
In terms of quality of life, the AXI-CEL treatment group and the SOC therapy group have 46 and 42 patients who are satisfied with life therapy, and on the 100th and 150th day, the patient's EORTC life quality measurement table and physical functional evaluation are evaluated. With the EQ-5D-5L VAS meter, the evaluation results support the quality of life in the AXI-CEL treatment group than the SOC therapy group, and the difference is clinically significant. In the 15 months of overall evaluation, the quality of life of the SOC treatment group has never reached the level of assessment of the baseline.
In general, compared to second-line SOC treatment, patients with R/R LBCL, ≥65 years old, received AXI-CEL treatment to significantly improve EFS and security. At the same time, Axi-Cel's comparison of SOC has also significantly improved the quality of life of patients.
3. CAR-T cell cocktail therapy for CD19 and CD22 for humanization of CAR-T cells (R/R) B cells non-Hodgkin lymphoma
Summary number: S213
Among the R/R B cells are not Hodgkin lymphoma, CAR-T cell therapy targeting CD19 shows a 50%-60%response rate. However, immune escape recurrence is one of the main challenges of long -term tumor control. In previous studies, the two-target CAR-T cocktail therapy targeting CD19 and CD22 can reach 93.3%in R/R lymphocyte leukemia, and can reduce the risk of CD19 negative recurrence.
This phase I/II study explored the efficacy and safety of CAR19/22-T cell cocktail therapy to treat R/R invasive B-cell lymphoma. From July 2020 to December 2021, the study was included in patients with patients with R/R invading B-cell lymphoma. All patients received Freidalabin 30 mg/m2/d and cyclopension 250mg/m2/d for 3 consecutive days (-5 days to -3 days), and then received CAR-T cell cocktail therapy.
A total of 26 patients received CAR19/22 cocktail therapy, the median age was 46 years (4-75 years old), and received the treatment of medium-level 3 (2-5 line). Among them, 4 are Pibkit lymphoma, 2 -bit filtering lymphoma (3A), and the rest are DLBCL patients. In addition, 7 patients were dual-strike lymphoma, and 4 patients recurred after receiving CD19 CAR-T cell therapy before.
14 patients received a rat-T cell therapy for rats, and the median dose was 2.18 × 106/kg (1-3 × 106/kg) CAR19+1.75 × 106/KG (1-3 × 106/kg) CAR22; Patients receive human-source CAR-T cell therapy, with a dose of 2 × 106/kg car19+5 × 105/kg car22.
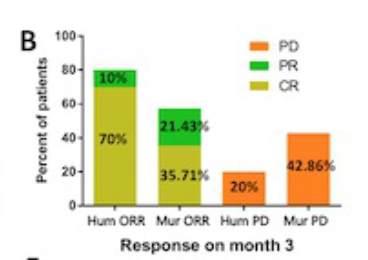
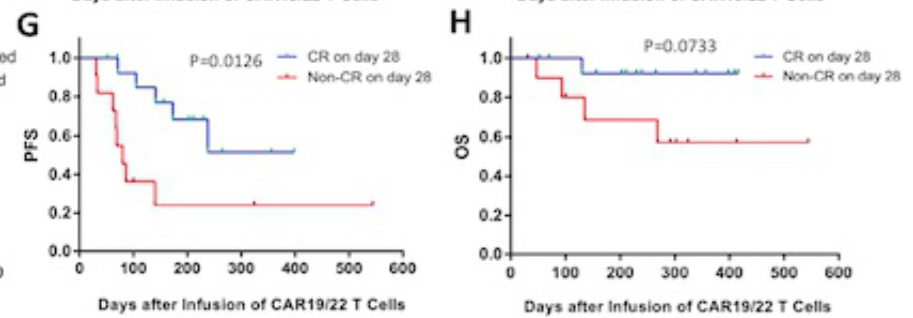
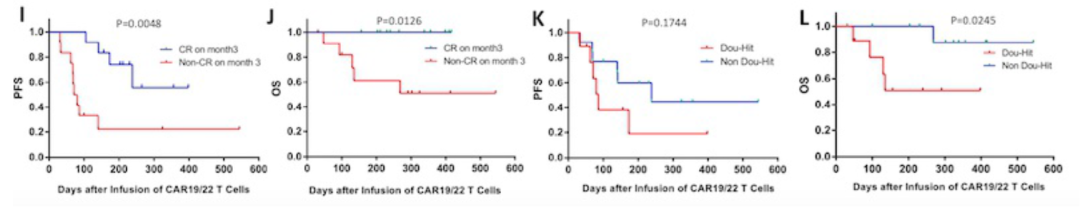
On the 28th day, the patients who were treated with human-source CAR-T realized all responses, and 9 of them CR, and 3 patients reached partial relief (PR). The ORR and CR rates of the human source CAR-T group were 85.71%and 42.86%, respectively.
4 patients with clinical trials in the group have achieved CR. In the third month of the assessment, 70%of the patients received CAR-T the treatment of patients still reached CR, while the CAR-T treatment group of Rat-T therapy group was only 35.71%.
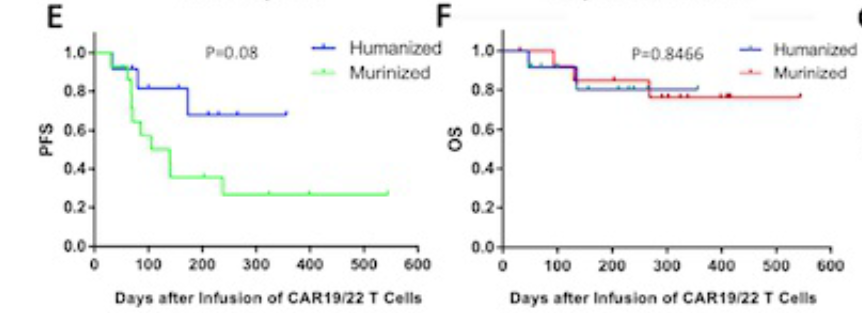
The median follow-up time is 291 days (31-544), and the 1-year PFS rate of the human CAR-T group is higher than that of the Rat source CAR-T group, (67.9% vs 26.8%, P = 0.08).
After analysis, CR (HR 0.21, 95% CI 0.06-0.72; P = 0.012) and until the third month still maintain CR (HR 0.18, 95% CI 0.06-0.5; P = 0.004) is the extension of PFS to extend the PFS Independent prediction factors. The third month maintains CR (HR 0.10, 95%CI 0.01-0.61; P = 0.012) and non-dual-strike lymphoma (HR 0.11, 95%CI 0.02-0.76, P = 0.024). In terms of safety, most patients have mild CRS (level 1-2), only two patients have a CRS of 3, and two patients have 3 levels of neurotoxicity.
4. Use the new targeting CD19 CAR-T cell therapy (ANBAL-CEL) to treat PD-1 and TIGIT's non-answering R/R LBCL phase I/II clinical research
Summary number: S214
Anbal-Cel is an autologous CAR-T cell therapy that targets the 2nd generation of PD-1 and TIGIT. Compared with the traditional CD19 Car-T, Anbal-Cel is better or in vitro or internal body. In terms of mechanism, knockouts for CAR19-T cells PD-1 and TIGIT can delay the exhaustion of CAR-T cells.
Patients are packed according to dose level 1 (2 × 105 cells/kg), dose level 2 (7x105 cells/kg) or dose 3 (2x106 cell/kg). The lymphocytes were removed with cyclumamide and fluulabin, and Anbal-Cel was inferred after 3 days.
As of January 17, 2022, nine-bit R/R DLBCL patients received Anbal-Cel treatment, and 4 of them received dosage 1, 3 grades of dosage 2, and 2-bit receiving dose 3. The median age is 54 years old (scope of 26-71 years); all patients have received 2 and above treatment, and 44%of patients have received ≥4 treatment. 78%of patients recur after the last treatment. 67%of patients have a IPI score of 3-4, and 44%of patients are accompanied by large blocks.
It was found that no patient had dose restrictions. Among the 9 patients, 5 CRS occurred, 4 of which CRS was 1-2, and 1 level 3 CRS occurred. The median occurred 7 days (1-16 days), and the median duration was 4 days (0-18 days).
Patients with 1 -bit dose 3 experienced level 2 ICANS, which occurred on the 7th day and lasted 13 days. The patient had a history of cumulative diseases. The most common level 3/4 AES is the decrease in neutral granulocyte counts (67%), anemia (56%), platelet decrease (22%), and platelet counts decreased (22%), and no infection AE occurs. The CR ratio is 78%, and it is fully relieved in patients with the lowest dose and immunohistochemistry (IHC) displaying CD19 <10%.
CRC01, which can be observed, expresses the dose -dependent CRC01. The dose -level group 1 group, dose 2 group, and dose 3 group medium -level drug concentration reaches peak time (TMAX) is 15.4, 15.8, and 11 days, respectively. The median peak concentration (CMAX) There are 18003, 30103, 53688 copies/μg GDNA, and AUC0-28 of CAR-T are 679125, 1110108, 2852235 copy/μg GDNA.
Anball -cel shows good curative effect and tolerance safety in this dose -increasing study. Subsequent clinical studies will evaluate the relief rate of CRC01, relieve duration and safety, and explore biomarkers.
5. Use T-CARGE's CAR-T cell therapy (YTB323) to treat the i-stage data results of R/R DLBCL
Summary number: S212
It can be seen for details (China and the United States "Two Worlds": Talking about the status and outlook of CAR-T from 2022 EHA)
Frontier of the tumor you want
Please pay attention to the doctor station 注
1. Scan the QR code below
2. Click to download the app

3. Open the doctor station app and click on the upper right corner

4. Find the tumor in the channel
And follow
Download the doctor's station app and subscribe anytime, anywhere ~
The first release of this article: blood channel in the medical community
Author: oolong tea

Review of this article: Wu Huijing, Hubei Cancer Hospital
Editor in charge: Sweet
- END -
End with flowers!"Internationalization around" Shenzhen City Exploration Activities Harbin Institute of Technology (Shenzhen) Station is successfully ended ~

The following article comes from the Shenzhen International Exchange and Cooperati...
Dongguang, Hebei: Drama enters the rural culture to benefit Wanjia

In order to vigorously promote the excellent traditional culture of China and enri...