For newly approved certificates for RELERE, women tumor patients ushered in the new choice of "Chinese characteristics"
Author:Cancer Channel of the Medical Time:2022.07.11
*For medical professionals for reading reference
Professor Wang Changyu explained the background and significance of the approval of the new indicator of the Rayleyzab!
Cervical cancer, endometrial cancer, and ovarian cancer are the three major gynecological tumors that seriously threaten women's life. In recent years, the advent of immunotherapy has improved the survival and quality of life of multiple tumor patients, including patients with maternal tumor. At present, in gynecological malignant tumors, immunotherapy has been recommended in the national comprehensive cancer network (NCCN) uterine endometrial cancer and cervical cancer guidelines [1-2].
On March 11, 2022, the PD-1 inhibitor of the PD-1 inhibitors of Baiji Shenzhou officially obtained the approval of the China Drug Administration (NMPA) for Rayleyzab, which was used to treat the previous menstrual governance and partial late stage of MSI-H/DMMR entities Tumor patients. What is the significance of this approval to the field of women's tumor diagnosis and treatment? The "Medical Circular Cancer Channel" invited Professor Wang Changyu of Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology to share the current status of the diagnosis and treatment of gynecological tumors and the current status of immunotherapy.
Women's tumor is worth looking forward to, and it is expected to become a new "main force" for women's tumor treatment
Professor Wang Changyu introduced: "Endometrial cancer, cervical cancer, and ovarian cancer are the three major malignant tumors that seriously threaten women's health. Traditional treatment methods include surgery, chemotherapy and radiotherapy. In recent years, the rapid development of targeted therapy and immunotherapy. Some breakthroughs have also been achieved in the field of gynecological malignant tumors. For example, the application of PARP inhibitors in the treatment of advanced ovarian cancer in advanced ovarian cancer significantly improved the prognosis of advanced epithelial ovarian cancer. The application of cervical cancer has also improved the survival of patients. As the immune examination point inhibitor continues to study in the field of tumor treatment, more and more clinical application research in gynecological malignant tumors has also been increasing. progress.
Immunotherapy of endometrial cancer
In recent years, research has found that the proportion of PD- (L) 1 in endometrial cancer is relatively high. Among them, the expression ratio of endometrial-like cancer PD-(L) 1 is as high as 40%to 80%, and transparent cell carcinoma is about 23 23 %~ 69%, the slurry cancer is about 10%~ 68%, and the incidence of mSI-H/DMMR in endometrial cancer is 37%to 40%. ICI) Higher gynecological malignant tumors benefit [3].
In terms of immune single medicine, the KEYNOTE series of studies have shown that Paborzab's treatment of patients with advanced or recurrence of endometrial cancer after at least one standard chemotherapy, of which the objective relief rate (ORR) of patients with MSI-H/DMMR is 53% ~ 57%, TMB-H patients are 46.7%, and PD-L1 positive patients are 13%. In addition, Garnet studied the efficacy of Dostarlimab-Gxly for platinum-containing progress or MSI-H/DMMR endometrial cancer patients. The results showed that ORR was 44.7%, and the disease control rate (DCR) was 57.3%. These studies support the treatment of PD-L1 monoclonal MSI-H/DMMR for the treatment of advanced and recurrent uterine endometrial cancer. [4-6].
In terms of immune combined therapy, the Keynote-146 evaluated the efficacy and safety of Paborizumab combined with lumininibinib for patients with late second-line chemotherapy and can be measured. The ORR of PMMR patients is 37.2%, MSI-H/DMMR patients are 63.6%, the mid-bit relief duration (DOR) is 21.2 months, the median PFS is in July, and the median OS is 16.7 months. MSI-H patients have significant effects among patients with MSS, and they are also better than single-drug treatment among MSS patients; Keynot-775 III studies also show that Paborzab's anti-combined luminibinib the treatment has received at least a late late recurrence of platinum chemotherapy in the past. Patients with endometrial cancer have extended PMMR patients with 2.8 months PFS and 5.4 months of mid -position OS compared to chemotherapy, and ORR increased by 15.2%. For the overall population, the median PFS extended 3.4 months. %, The median OS extended for 6.9 months, ORR increased by 17.2%. The guide is based on this recommendation of Paborizumab Lianlunkinib for MSI-H/DMMR patients with advanced recurrence of endometrial cancer [7-8]. Immunotherapy of cervical cancer
Early cervical cancer patients can get better prognosis through surgery, radiotherapy and chemotherapy. However, the treatment of advanced recurrence of cervical cancer is always difficult to treat, and the efficacy needs to be improved. The proportion of patients with MSI-H/DMMR among cervical cancer patients is low, only about 2.26%, but the proportion of PD-L1 positive patients with cervical cancer patients is as high as 34.4%, and there are even 96%of literature reports. This prompts PD-1 suppression The agent is expected to be used for the treatment of advanced cervical cancer. At present, immunotherapy has made certain progress in the field of cervical cancer. The clinical trials of immunotherapy combined with chemotherapy, radiotherapy, PARP inhibitor, TKI drugs and other clinical trials in the treatment of cervical cancer are underway, and some research has achieved considerable effects.
As far as immunotherapy is concerned, in the Keynote series, the ORR of Paborizumab treatment PD-L1 positive patients is 14.6%to 17.0%. Another several research results show that the ORR of the late -stage/recurrence cervical cancer of Nawuli is 4.0%to 26.3%. The research on Keynote-158 and JAPICCTI-163, and 212 conducted a sub-group analysis based on the patient's PD-L1 expression. The results showed that PD-L1-positive people responded better to PD-1 inhibitors. In addition, EMPOWER/GOG-3016/ENGOT-CX9 III Studies have shown that compared with single-drug chemotherapy, Simi Pelidab can significantly improve the OS of patients with advanced metastatic cervical cancer after the advancement of platinum chemotherapy. And it has nothing to do with the type of expression of PD-L1 and the type of organizational science. Simi Pully Micide has reduced the risk of recurrence/death by 25%.
As far as immunotherapy is concerned, the KEYNOTE-826 phase III study explores the efficacy and safety of Paborizumab+platinum chemotherapy ± Bevarzab the treatment of recurrence of recurrence of cervical cancer. The results of the study show that compared with the control group, regardless of the expression of PD-L1, combined treatment can significantly increase PFS (10. April VS 8. February, HR = 0.65) and OS (24. April vs 16. May, HR = 0.67) [ 6], in 2022 NCCN cervical cancer guidelines have been recommended based on this advanced recurrence and metastatic cervical cancer in the first -tier treatment of Paborizumab combined with the first -line treatment, which laid the position of the first -line therapy of immunotherapy in cervical cancer [1].
Immunotherapy of ovarian cancer
As far as ovarian cancer is concerned, most ovarian cancer is pulp epithelial ovarian cancer, and the proportion of MSI-H patients is low. It is reported that only 1.37%are reported, TMB-H patients also account for only 1.47%. %~ 30%. Therefore, the efficacy of immune examination point inhibitors in ovarian cancer is limited.
In terms of immune single drugs, the 200 studies of Ninja and Javelin Ovarian suggest that the treatment of immune examination point inhibitors single drugs have not improved the prognosis of patients with ovarian cancer compared to chemotherapy, and the overall response rate is not high. At present, based on Paborzab's applications for patients with DMMR/MSI-H and TMB-H physical tumors, only Parbilizumab is clinically recommended to treat with DMMR/MSI-H or TMB-H. Treatment of patients with relapse ovarian cancer.
In terms of immune combined treatment, in terms of combined chemotherapy, the Javelin Ovarian 100 studies explored the effects of paclitaxel + card platinum chemotherapy combined with AVELUMAB to treat patients with initial ovarian cancer and used AVELUMAB to maintain treatment. The PFS benefits were not displayed, and the study was suspended for some reason. For platinum resistant recurrence ovarian cancer, some research of some immunochemical chemotherapy showed that the ORR was improved, but the response time was short. Based on this, it is not recommended that immunocardiac inspection point inhibitors combined with chemotherapy. As far as the combined targeting drugs are concerned, there are currently only research results of phases I and II, and the overall ORR is 15%to 32%. As far as the combined PARP inhibitor is concerned, MedioLa research shows that Ora Pali+Dagatuyu treats GBRCA mutations and platinum sensitive recurrence ovarian cancer patients for 71.9%, but Topacio/Keynote-162 Study in Nichapa The ORR of Paborzab's treatment of platinum resistant ovarian cancer is only 18%. Based on the above studies, although the immune checkpoint inhibitor combined with certain targeted drugs shows certain effects, it is not enough to recommend clinical applications. Professor Wang Changyu talked: "Generally speaking, immunotherapy has shown a certain clinical effect on some gynecological malignant tumors. Currently among the three major gynecological malignant tumors, immunotherapy is the best effect in endometrial cancer, followed by cervical cancer, followed by cervical cancer. The most effective effect of ovarian cancer. In addition, it is important to screen for the advantages of treatment before the application of ICI treatment. At the same time, the combination of ICI and chemotherapy, antiovascular generation treatment, PARP inhibitor and other combination are expected to improve the efficacy. "
Study in line with national conditions, innovative pharmaceutical structure, and brings practical benefits to women tumor patients for Rayleyzumab
Professor Wang Changyu said: "It is worth noting that although the treatment plan based on immunotherapy has shown considerable effects in the field of gynecological tumors, the lack of data for Chinese patients in previous related research It may be relatively limited.
Rationale-209 Research is the first domestic PD-1 monoclonal anti-anti-anti-anti-anti-treatment study in my country MSI-H/DMMR gynecological tumor patients. The effectiveness and safety of patients with DMMR physical tumors were announced at the 2022 American Gynecological and Tumor Association (SGO) conference.
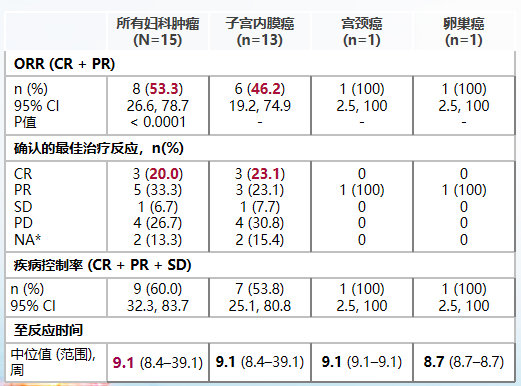
Ratchale-209 studies incorporated 80 patients with local advanced periods, mSI-H/DMMR physical tumor patients, 17 of which were patients with maternal tumor (15 cases of endometrial cancer, 1 case of cervical cancer, and 1 ovarian cancer). The results of the study showed that among the evaluated patients with gynecological tumors, the objective relief rate (ORR) was 53.3%, the (CR) rate was completely relieved by 20%, and the disease control rate (DCR) was 60.0%. Among them, the orr patients with endometrial cancer are 46.2%, the CR rate is 23.1%, and the DCR is 53.8%; and patients with cervical cancer and ovarian cancer also obtain the efficacy of partial relief (PR) [7].
Table 1. Rationale-209 research efficacy data
In addition, the median to the relief time (TTR) of Rayleyzumab was 9.1 weeks, and the treatment was faster. As of 17.5 months of the median follow -up time, the mid -level relief duration (DOR), PFS and OS of the women's tumor queue were reached. In terms of safety, the patient's overall tolerance and adverse reactions are controllable, and no new security signals are found [9].
Talking about the uniqueness of the Rayleyzumab, Professor Wang Changyu introduced: "The unique drug structure design of the Rayleyzaba makes it longer half -life and can maintain longer in the body cycle. Therefore Stronger anti-tumor activity. In addition, the FC section of traditional PD-1 monoclonal antibodies can cause antibodies to dependent cell phagocytopenic effects by combining macrophages and NK cells, which can cause T cells Losses, and the FC segment of the Rayleyzab's antibody part was transformed, which eliminates the ADCP effect of the Dearzumab to the greatest extent, and avoids the effects of T -cell losses against the treatment of tumor treatment. At the same time, its antibody FAB The segment and the PD-1 binding site are large, and the affinity of PD-1 is higher, which can more completely block the combination of PD-1 and PD-L1.
Professor Wang Changyu concluded: "Because the Rationale-209 research on the Rayleyzab is aimed at the research of Chinese patients, it is more in line with my country's clinical practice, so it has a strong guiding significance. It is also based on this research. Lizabu was approved in my country for the treatment of previous menstrual governance, and patients with advanced mSI-H/DMMR solid tumors in China and could further benefit our patients in my country. "
Expert Introduction
Professor Wang Changyu
Three -level professor, chief physician, doctoral supervisor

Huazhong University of Science Tongji Hospital, Tongji Medical College
Secretary and Deputy Director of the Party Branch of Gynecology Oncology
Standing Committee Member of the Chinese Medical Association Gynecology Branch
Member of the Hubei Medical Association Gynecological Tumor Branch
Member of the Obstetrics and Gynecology Branch of Wuhan Medical Association
Cancer Prevention Research Magazine Editorial Committee
Deputy Chairman of Hubei Gynecological Oncology Control Expert Committee
Hubei cadre health care expert
He presided over and participated in a number of National Natural Science Foundation of China, published more than 30 SCI papers, and participated in the editor of modern obstetrics and gynecology science, clinical obstetrics and gynecology science, gynecological tumor diagnosis and treatment experience. The research and application of its obstacle strategy won the second prize of national science and technology progress. The amount of surgery for many years is in the top ten references of the whole hospital
[1]. NATIONSIVE CANCER Network.nccn Clinical Practice Guidelits in onCology-Uterine Neoplasms.version 1.2022.
[2]. NATIONSIVE CARANCER Network.nccn Clinical Practice Guidelits in onCology-CERVICAL.Version 1.2022.
[3].Liu J, Wang Y, Tian Z, et al. Multicenter phase II trial of Camrelizumab combined with Apatinib and Eribulin in heavily pretreated patients with advanced triple-negative breast cancer. Nat Commun. 2022 May 31;13(1) : 3011.
[4] .le DT, URAM JN, WANG H, ET Al. PD-1 BLOCKADE in Tumors with Mismatch-Repair deficience. N English. 2015. 372 (26): 2509-20.
[5].O'Malley DM, Bariani GM, Cassier PA, etal. Pembrolizumab in Patients With Microsatellite Instability-High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study. J Clin Oncol. 2022 Jan 6:JCO2101874.
[6].Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S, Piperdi B, Mehnert JM. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study J clin oncol. 2017 DEC 1; 35 (34): 3823-3829.
[7] .makker v, colombo n, casado herráez a, et al. Lenvatinib plus pembrolizumab for advanced endometrial.
[8].Matthew H. Taylor et al. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J Clin Oncol 2020.
[9] .Dong Wang, et al. 2022 SGO. Abstract #127.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
558 newly infected people a week, and 91 high -risk areas with a population of less than one million

The People's Daily Health Client learned from the small program inquiry of the Sta...
Winter disease summer treatment!Sanfu moxibustion, Sanfu post, Sanfu can, Sanwu scrape, there is always one suitable for you

Sanfu Tian is the highest temperature and humid and sultry period in the year. Dur...