Baiji Shenzhou Wanglai: Innovative medicine goes to the sea, you have to break a way to walk through no one
Author:Kenji Bureau Time:2022.06.28

How difficult is China's "innovative medicine" to be recognized by the world?
After a new round of medical reform in 2009, the domestic pharmaceutical industry made a momentum to catch up with the world's pharmaceutical innovation. With the support of policies and funds introduced one after another, "going overseas to do clinical" has become a trend of the pharmaceutical industry in that era.
In today's perspective, an innovative medicine does not conduct one or two international multi -center trials, and it is almost unlikely to be internationally recognized. But more than ten years ago, it was very amazing to go to the world to launch clinical practice. At that time, domestic innovative drugs carried out internationally clinical, and there were two more representative drugs: 1 new drug "Reorganized New Lam Green" with heart failure, and "Sidamonamine" for the treatment of cancer.
The pre -clinical study of these two drugs was launched in 1999 and 2003, respectively. It was considered to be grinding a sword for ten years. It was once highlighted. Scientists invented the two drugs were also tough and persistent. But unfortunately, the two medicines are not going smoothly.
The reorganized person in Nuramus had a patented layout in the United States, the European Union, and Japan, but has not been able to make good clinical results. In early 2020, it was even rejected by the State Drug Administration. This amine was approved in China in 2013, but it was not obtained by Japan's listing permit until 2021.
How many scientists can there be a new drug in the international market in the lifetime of ten or twenty years?
In fact, before 2019, none of the innovative drugs in China can obtain a listing license to the US FDA. For all Chinese scientists, this is a competition that has never had experience.
This is even more so for Wang Lai. Different from many people who have been immersed in large multinational pharmaceutical companies for many years, before joining Baiji Shenzhou in 2011, Wang Lai did not have worked in multinational pharmaceutical companies. In his own words, it was a "wild road". But it is such a person in charge of R & D that does not follow the usual way that leading a young team to create a record in the Chinese pharmaceutical industry: the first to send domestic innovation anti -cancer drugs to the US market.
In November 2019, Zibuti, independently developed by Baiji Shenzhou, was approved by the US FDA, becoming the first new anti -cancer drug in the United States in China to achieve a "zero breakthrough" for new drugs. In the first quarter of 2022, Zibutini sales in the United States reached 431 million yuan, a 7 -fold increase year -on -year. In June 2022, Zibuti has been listed in 50 countries and regions around the world, and is basically a foothold in the world market.
The development of new drugs is a competition for technical strength, and it is a strategic competition. The R & D layout and talent echelon design of Baiji Shenzhou allows the company's products and pipelines to be in a very favorable competitive pattern, which is inseparable from Wang Lai who "does not follow the routine".
Stop the pressure and go to the international arena
In 2011, Wang Lai followed the tutor Wang Xiaodong to join the newly established Baiji Shenzhou. Wang Lai, who was born in the scientific research community, did not have the experience of large multinational pharmaceutical companies. At that time, he did not know what kind of company Baiji Shenzhou would become a company. But one thing is clear: this is a "new generation" biotechnology company, which must be different from other biomedical companies.
In 2012, the BTK inhibitor Zibntinib was at the beginning of the project that Wang Laili mainly promoted the project established by the company; this also projected the early research and development ideas of Baiji Shenzhou.
When Zhebutoni was settled, the first generation of BTK inhibitors in the world had entered the clinical phase 2. However, after repeated research by internal teams, Wang Lai and other scientists saw that Ibetinib still has a lot of room for optimization. Therefore, the team firmly believes that there is a great opportunity for the development of a new generation of BTK inhibitors.
"The first product to hit the line often provides a lot of experience and lessons for future generations. Even if the first collision is successful, it is often not perfect and can provide us with a lot of reference. Drugs. "Wang Lai concluded the development idea of a new generation of BTK inhibitors.
In the field of new drug research and development, this direction of R & D is called "Best-in-CLASS", which is pursuing continuous improvement and later. As the representative of the innovative pharmaceutical enterprise, Baiji Shenzhou did not seek first, but chose areas that are easier to break through and make differences. It sounds less tall, but in the early days of the company's entrepreneurship, this strategy is very effective.
Zabitinib was the first innovative drug developed by Wang Lai, and many "extra" work naturally fell on his head.
After completing the laboratory development, Zibutinib faced the problem of carrying out clinical trials of human body. However, in 2013, the approval of the domestic new drug clinical application at that time was slow. In order to accelerate the development, Baiji Shenzhou decided to carry out international clinical clinical clinical. At that time, the team's "lack of experience" and "lack of people", Wang Lai, who was originally responsible for preclinical research, could only hold his head hard. Wang Lai and the team chose to go to Australia to find a way to find local experts, visit the door one by one, and draft and design clinical trials with experts.
The overseas clinical, which was initially carried out for "rushing progress", gradually formed a differentiated path, which became the "standard" of the company's new drug development. Wang Lai believes that since we firmly believe that we can make the best anticancer drugs in the world, we should set higher goals to benefit patients around the world. Focusing on this idea and development path, from 2013, a number of products in Baiji Shenzhou have been quickly pushed into the clinic at home and abroad.
In fact, clinical trials overseas spend money, and due to the high loss brought by a large number of global clinical trials, it has always been the target of the outside world that does not understand and criticize the Baiji Shenzhou. Wang Lai frankly said: "Answer these questions have become part of my work. However, even if we are under these pressures, we must do this. We firmly believe that although the road of internationalization is difficult, this is the way out of innovative medicines, and China is even more in China. The way out of the pharmaceutical industry. "
Wang Lai has its own judgment on China's and global medical patterns: China is indeed a large population country, but the domestic economic development requires a process. The pharmaceutical industry is based on the needs of people's livelihood. Essence To cultivate a great global pharmaceutical company on this soil, we must move towards the international market.
Create a global R & D machine with a strict seam
It is far more difficult to form a global R & D team from scratch.
During the period when Baiji Shenzhou was just founded, China has not formed a new drug research and development atmosphere, and it lacks experienced scientific research talents. Moreover, Baiji Shenzhou was a small company at the time. Scientists in Chinese foreign pharmaceutical companies at that time were unlikely to see such a startup.
Wang Lai divided the research and development of Baiji Shenzhou into two stages. Before 2016, many of the company's pipelines were mainly in the laboratory stage; after 2016, key work began to concentrate on the clinical end. After 2015, when China started the reform of the pharmaceutical and administrative system, the speed of approval of new drugs has increased significantly, bringing a group of innovative pharmaceutical companies in China to bring "counterattack" opportunities.
In order to set up a Chinese clinical team first, Wang Lai spent a long time recruiting soldiers in the industry. Among them, there were many people dug from foreign pharmaceutical companies. The research and development functions of foreign companies in China are mainly clinical research. However, in the past, China rarely intervened and participated in the formulation of global drug development strategies. It is often planned to implement plans from global headquarters. It is far from the decision -making layer. The ceiling is very obvious.
In order to attract more people to join Baiji Shenzhou, Wang Lai started to "tell stories" over and over again, tell his views on the industry, talk about the spring of Chinese innovative medicines, and tell the historic opportunities of creating a career. Also tell the headhunter.
Wang Lai has always emphasized: Baiji Shenzhou shows a piece of white paper to colleagues. There is no program, no routine, and countless people who need to join the company to help it become an efficient and unique company.
回忆起早期搭建团队的经历,汪来当时最大的感受就是:“在百济神州,你不得不撸起袖子去干本来或许不该你干的事情,因为公司需要有人做这些事,而且必须做Okay. This is a kind of master spirit that we always emphasize. This experience is also the biggest improvement in my career. The more we do, the more the plates are. At that stage I am more familiar with our clinical. "
Until the beginning of 2019, the Asia -Pacific development team with the Chinese clinical team was basically formed, and Wang Lai turned to form a US clinical development center.
In the R & D system of Baiji Shenzhou, pre -clinical research is mainly deployed in China, while clinical development is a "dual -core" structure, which is equal to China and the United States. At the same time, it will deploy clinical operations and pharmaceutical registration in other parts of the world. Let the global clinical team and the Asia -Pacific clinical team perfectly combine the advantages and ability, and it is necessary to shoot with the pre -clinical research team to build a global R & D engine that operates efficiently. To this end, Baiji spent 6 years.
Due to the different cultural background, China's R & D team is often more efficient, while the rhythm of overseas teams is relatively soothing. The operation of the two gears is often not on a beat, and it needs to be more closely running -in. "How to make domestic and foreign teams cooperate on the same channel to become a global One Team, which is essential for us to build a global R & D system, but this is also particularly challenging." Since 2019, Wang Lai has gradually gradually gradually gradually gradually become Responsible for the management of the American clinical team. After several years of polishing, the two major gears were completely closely combined.
Today, the size of this clinical development team that spans many places around the world has exceeded 2,100, becoming the largest clinical development team in the global tumor field, and gets rid of the dependence on third -party CRO institutions. In your own hands. Although the process is "a lot of effort", this layout is intended to ensure the quality of clinical trials to a large extent, accelerate development progress, and reduce costs.

"Now I am more confident than before any time." Wang Lai, who has become the person in charge of the global R & D of Baiji Shenzhou. When talking about the future, I hope that the company can maintain the vitality as a biotechnology company for a long time. "With this one The efficient operation of large engines, we can hatch more potential new projects inside the company. We used to do Best-IN-Class, and now the conditions are mature. We are already in the second wave of innovation. -In-Class fully exerts its strength. "
Wang Lai introduced that in more than 100 clinical trials that Park Ji has carried out worldwide, less than 30%of them are only launched in China, and most of the rest are international multi -centered clinical studies. There is a fundamental difference between the data composition and investment scale of clinical trials in China, and its purpose is to lay the foundation for the global declaration of more pipeline products. "In the future, every product we develop independently should have the potential of internationalization." It is interesting that the hundred Shenzhou, which once dug people from foreign -funded multinational pharmaceutical companies, has now begun to "dig people" by multinational pharmaceutical companies to "dig people". It's right. Wang Lai believes that as a team manager, it is very important to find "the right person" and use people in the right place. "Many things that Baiji Shenzhou do have no precedent in the industry. In this process, you must maintain a flexible environment, give people enough vitality in business, not only so that good talents can integrate, but also allow them to be able to allow them There are more space to explore. As a manager, we must be responsible for the career of the employees, design it for him, give him enough help, so that he has more opportunities to raise his ceiling. This is also my biggest. pleasure."
Good scenery is not only at the peak, but also at the trough
Baiji Shenzhou refused to become another "traditional" biomedical company. Along the way, Baiji Shenzhou did not follow the model of the company in the past. Instead, he went out of a unique business model and development path.
In the first ten years of starting a business, whether it is BTK inhibitor or PD-1, Baiji Shenzhou is still in the international running stage. But with the strength of the future, Baiji Shenzhou is gradually chasing the leader.
In the "Second Invoicing wave", Baiji Shenzhou hopes to become a leader. The biggest difference between leading and running is that the leading running needs to choose the correct direction. It is not simply a technical consideration, and it is also a test of strategy and patience.
Drug innovation is actually the leading business model. At present, the hottest PD-1 development story proves this. Scientists discovered the targets of PD-1 and PD-L1 as early as the end of the last century, and believed that they could develop unprecedented cell immune drugs. However, at the time, the targeting anticancer drugs had just risen and the commercial value was not completely excavated, so the value of PD-1 has not been fully excavated.
It was not until 2012 that the global "patent cliffs" arrived, and Mer Shadong and other multinational pharmaceutical companies turned out of PD-1 from the pipeline, accelerated the research and development and pushed to the market within a few years, and then the global pharmaceutical industry rushed.
If you want to be lighter, you can take out heavy varieties such as PD-1 at any time. Baiji Shenzhou needs to accumulate deep research and development pipelines, and slowly build a new drug research and development structure that meets the global innovation trend.
Wang Lai spent a few years of internal process transformation and power system reconstruction, which is the basis for the next round of new waves.
More importantly, in China to cultivate a pharmaceutical innovation ecosystem, and use mature business models to support this system. With the ability of the industrial value chain, the ability to change the global pharmaceutical industry is not efficient and disadvantaged. , Bring high -quality drugs to the world, and maximize the value of scientists, to maximize the energy of innovation -this is the core and ultimate purpose of setting off the wave of innovation.

Nowadays, the most important keywords in the domestic pharmaceutical industry are "innovative medicine cold winter". All parties try to explain the current industry changes from the aspects of policies, capital, and society.
Wang Lai believes that this round of industry bubbles have been brewed before the epidemic in 2020, but as the epidemic strikes, the pharmaceutical industry is pushed under the spotlight, allowing many people to ignore the risk existence. For a long time, homogeneous competition and fighting in the same market will only dispel the courage to move towards the unknown, and it will not make the industry better.
In fact, many times the real scenery is not only at the peak, but just in the trough. "The trough often gathers the industry together. Everyone can systematically reflect and reconcile, in order to twist into a rope, and find the direction to start again." Talking about the current innovative drug environment, Wang Lai is still optimistic about the future.
"There is no quick rule for doing innovative medicines, and we must adhere to long -termism. After a round of shuffling, I believe that the biomedical industry will enter a fast -rise channel again. From the global perspective, there are many problems with the improvement of human health and the improvement of quality of life. It is necessary to answer and solve science. In addition, the most important thing is that the technological innovation of the biomedical industry has finally reached the era of full flowering. With the breakthroughs and outbreaks of more and more technologies, more excellent biomedical companies will appear ","
Today's Baiji Shenzhou is accumulating for the next round of development opportunities. Wang Lai joked: Baiji Shenzhou just did the right thing at the right time, encountered countless good luck, and we all caught it. This is a lucky. But now we can't say that we have succeeded, everything is just the beginning, there are still long ways to go, and many things can be done.
"Innovative medicine is an opportunity to give it a good time. Why don't you run well?"
####
- END -
From 0:00 on June 24, 2022 to 24:00, there are no new local diagnosis cases in Shandong Province, and those who have no symptoms in the local area

From 0:00 to 24:00 on June 24, 2022, there were no new local diagnosis cases in th...
The IPO qualification of medical device enterprises is relaxed, 0 income can also be listed on the market
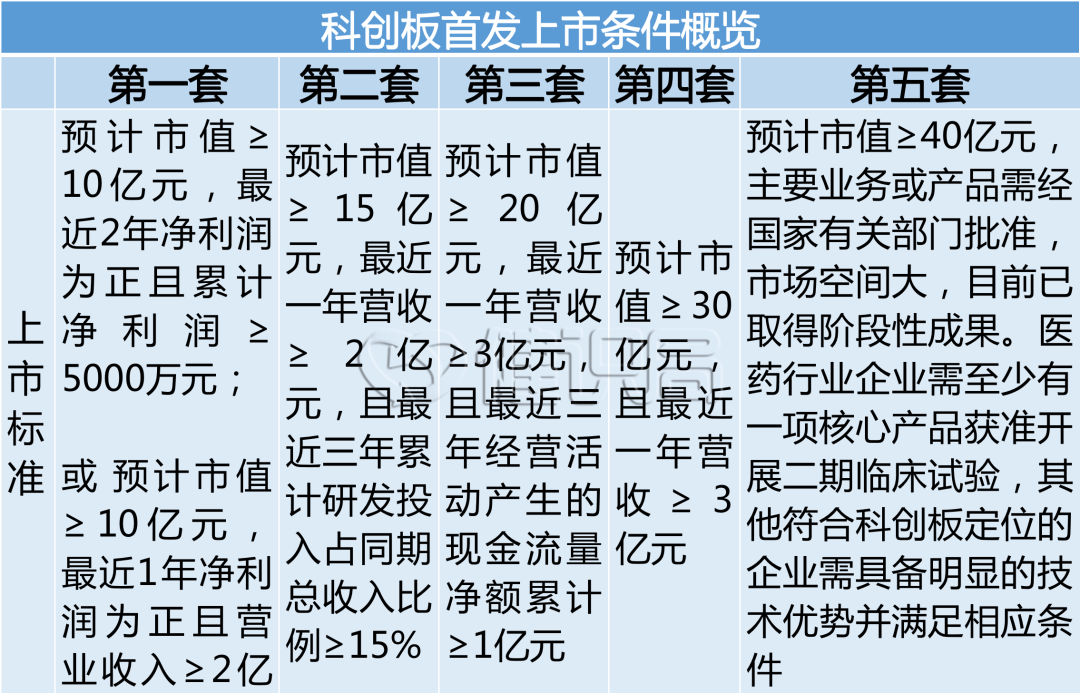
On June 10, the Shanghai Stock Exchange issued the Guidelines for the Fifth Settin...