Strive from the design: Lolatinib's innovative structure drives the efficiency upgrade, open the new chapter of ALK first -line treatment
Author:Cancer Channel of the Medical Time:2022.06.24
*For medical professionals for reading reference
Interpret Loladinib's innovative structure, brain protection strength and other clinical advantages.
On April 29, 2022, the third-generation inter-intercipate lymphoma kinase (ALK)-The TKI Loladinib was approved by the China Pharmaceutical Supervision Administration (NMPA). The treatment of patients with local advanced or metastatic non-small cell lung cancer (NSCLC) is also the first three-generation Alk-TKI approved in China.
In the context of China's lung cancer treatment as a whole, the progress of ALK integration is very rapid. The ALK-TKI of the three generations of the same hall has greatly enriched the sequential medication pattern. As an expert in the field of pharmacy, Professor Fang Luo, Zhejiang Cancer Hospital, is familiar with the structural characteristics and functional characteristics of one/two/three generations of Alk-TKI. The "medical community" invites Professor Fang Luo from the perspective of pharmacy to analyze the update iteration of Alk-TKI, as well as the efficiency upgrade and pattern change brought about.
Structural upgrade -Lolatinib broke through the routine and adopts an innovative large -cystamide structure
Alk-TKI, which has been approved to be listed, presented the "Three Generations of Tongtang", from the earliest generation TKI Cizininib to several second-generation TKIs such as Alantinib and Sedinib to three generations of TKI Lola Entinib, they all belong to the competitive inhibitors of tritenosyate (ATP). [1].
""
Professor Fang Luo said that during the iteration of ALK-TKI, the direction of new drug research and development includes improving the combination of drugs and targets and improving medicine. The side chain outside the pharmaceutical function group determines the absorption process, metabolic process, intracranial distribution, etc. of the drug in the body. The iterative update of the drug is usually modified for the side chain. the goal of. The first and second-generation ALK-TKIs are ringless and long-chain-shaped compounds. The third-generation ALK-TKI Lolatinib broke the routine of the drug iteration update, boldly innovated in the structure, and adopted a completely different small molecular large-cycleamide Structure [2].
"
Brain control upgrade -Lollantinib enters the brain strong, exerts the strength of brain protection
Small molecular macromide structures help Lolatinib can better control intracranial lesions while giving full play to the "soft power" of brain protection.
""
Professor Fang Luo pointed out that, compared with ALK genes without fusion of patients, ALK -positive NSCLC patients are more likely to have brain metastases. Therefore, TKI drugs are very important to penetrate blood brain barriers and treat brain metastases. In the past few years, most related studies have often "avoided" patients with brain metastases. The first generation of TKI Kazizyini has insufficient ability to penetrate blood -penetrating blood -threatening brain barriers. The second -generation TKI has been improved on this basis. Internal lesions are completely relieved (CR) ranging from 9%-45%[3-5], and the curative effect is still unsatisfactory.
The first and second -generation TKIs are ring -shaped and long -chain -shaped compounds, which leads to higher hydrophilicity and is not easy to pass through the blood -brain barrier. The main factors that affect the drug through the blood -brain barrier are the molecular weight, fat -soluble, etc. of the drug. Lolatinib adopts the large -cycleamide structure in design, which has better metabolic stability. Lasticity [2], this is conducive to reducing P-sugar protein (P-GP) to be mediated by the external discharge effect, enhances the ability of Lolatinib to cross the blood brain barrier and the ability to stay in the brain, and improve Treatment efficacy and brain protection effect of brain metastases.
"
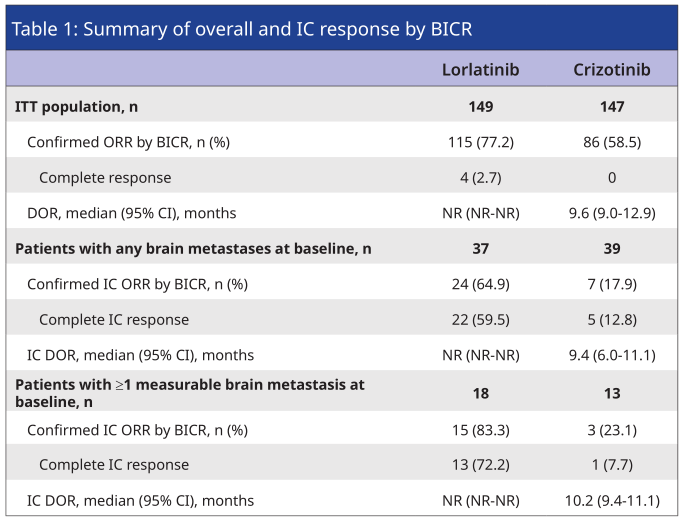
Based on the advantages brought by the unique large ring structure of Lolatinib, Lolatinib's strong brain strength was confirmed in the CROWN research results updated by the AACR Conference in 2022 [6]. Among the patients with ≥1 measuring brain metastases in the baseline, the intracranial objective relief rate (ORR) of the first -line treatment of the Lolatinib group reached 83.3%, and the intracranial CR rate was as high as 72.2%, which reflected the Lolaininini to ALK Positive NSCLC's comprehensive control ability. Among patients with no brain metastases, only one case occurred in the three years of follow -up, and only one case of intracranial progress occurred, and the survival rate of intracranial progress in 3 years was as high as 99%, which reflected the "soft softness of Lolatini for brain -free patients' brain protection" soft transformers. strength".
Table 1. Crown research in the craniotomy relief of BICR assessment
Unlimited vitality -enriched ALK -positive NSCLC first -line drug selection
In the past, the first-line treatment of patients with ALK-positive NSCLC was only one generation and second-generation Alk-TKI. Lolatinib's domestic approval was further enriched to ALK-positive NSCLC first-line therapy.
""
Professor Fang Luo said that in the CROWN study treated in the first line of Lolatinib [6], the Lolatinib group has followed up to 36.7 months but has not reached the end of the no progressive survival (PFS), and the PFS curve has not seen a downward. The trend is more than 60%of patients who have no disease progress or death. In contrast, the median PFS of the first-line treatment of the two-generation Alk-TKI is about 25 months or so for about 25 months. [7-9].
Lolatinib broke through the new PFS record of ALK's first -line treatment. Regardless of the baseline or without brain metastases, the first -line treatment of Lolatinib can bring significant medium -ranked PFS benefits. It is expected to rewrite the first -line treatment pattern of ALK positive lung cancer. Not only is the first -line treatment, Lolatini can also be used for back -line therapy of previous first/second -generation TKI resistance patients. It is a "guarantee" drug for patients with multi -line treatment failure. "
summary
The advantages of Lolatinib's large -ring structure have been well verified in the CROWN research, which has the advantages of strong effect and high blood brain barrier permeability. ALK -positive NSCLC patients were treated by Loladinib for the first time. The median PFS evaluated by BICR for more than 36.7 months has not yet reached the end point. Latuni is expected to become a preferred plan for front -line treatment. At present, Lolatini has been recommended by the 2022 version of the China Clinical Oncology Society (CSCO).
Expert Introduction
Professor Fang Luo

Director of the Department of Pharmacy, Affiliated Cancer Hospital (Zhejiang Cancer Hospital) at the University of China
Zhejiang "Ten Thousand Talents Plan" youth -top talent, 151 talent engineering training personnel in Zhejiang Province
Zhejiang Province Health Innovation Talent Training Objects, Rookie of Zhejiang Province
Academic:
Member of the National Cancer Quality Control Center Pharmaceutical Quality Control Commission
Standing Committee of the China Anti -Cancer Association Cancer Clinical Pharmacy Commission
Member of the China Anti -Cancer Association Anti -Cancer Drug Special Committee
Member of the China Hospital Association Pharmaceutical Commission
Deputy Chairman of the Zhejiang Anti -Cancer Association Anti -Cancer Drug Special Committee
Deputy Chairman of the Zhejiang Pharmaceutical Society Pharmacy Specialty Committee
Zhejiang Mathematical Medicine Society Smart Pharmaceutical and Reasonable Drug Special Committee Deputy Chairman
Youth Chairman of the Zhejiang Pharmaceutical Society of Pharmaceuticals
Youth Deputy Chairman of the Zhejiang Pharmaceutical Society of Pharmaceuticals
Member of the Zhejiang Pharmaceutical Society Hospital Pharmacy Commission/Clinical Pharmacy Commission
Director and academic work of Zhejiang Pharmacological Society
Prested 2 projects on the National Natural Science Foundation of China and 1 key R & D project of the Zhejiang Provincial Department of Science and Technology
First/Communication Author Published 30 SCI Paper Articles 30 Articles
references:
[1] Song X, Zhong H, Qu X, Yang L, Jiang B. Two novel strategies to overcome the resistance to ALK tyrosine kinase inhibitor drugs: Macrocyclic inhibitors and proteolysis-targeting chimeras. MedComm (2020). 2021;2(3 ): 341-350.
[2] Johnson Tw, Richardson PF, BAILEY S, et al. Discovery of (10R) -7-Amino-12-Fluoro-2,10,16-Trimethyl-15-Oxo-10,15,16-testrahydro -2H-8,4- (Metheno) Pyrazolo [4,3-h] [2,5,11] -BenzoxadiazacyClotetradecine-3-CARBONITRILE (PF-06463922), A MacroomClic inchiblic Lymphomakomase ( ROS Oncogene 1 (ROS1) With Preclinical Brain Exposure and Broad-Spectrum Potent ALK-Resistant Mutations. J Med Chem. 2014; 57 (11): 4720-4744.
[3] Peters s, Camidge Dr, Shaw at, et al. Alectinib versus crizotinib in Untreated Alk-POSITIVE NON-Small-Cell LUNG CANCER. N ENGL J Med. 2017; 377 (9): 829-838.
[4] SORIA JC, TAN DSW, Chiaari R, et al. FIRST-LINE CERITINIB Versus Platinum-Based Chemotherapy in Advanced Alk-Rearranged Non-Small Lung Cancer
- END -
After reading these rumors, I almost dare not drink water

One minute of Ph.D., the posture continues to rise-The end of this issue-
The latest judgment of the Beijing epidemic situation!

At 16 o'clock on June 18, Beijing held a press conference of the 369th crown epide...