Who can cross the cycle?"Legend" of China CAR-T therapy
Author:Medtrend medical trend Time:2022.06.23


Legendary creature was founded in Nanjing in 2014 and was incubated by the "world's largest genetic synthesis" supplier Kings Rui. At the beginning of his business, the legendary creature was in Nanjing, and he did research silently and was unknown.
What really made the legendary creature "breaking the circle" was to reach a heavy cooperation with the multinational giant Johnson & Johnson in 2017.
At the end of February 2022, CARVYKTI, a legendary creature targeting BCMA, officially received FDA approval, is the first domestic CAR-T product to be listed in the United States. This has an epoch -making milestone for the cell gene therapy industry.
The goal of the legendary creature is to treat the potential of those who are considered to be incurable, such as blood malignant tumors, physical tumors and infectious diseases through cytotherapy.
Development History
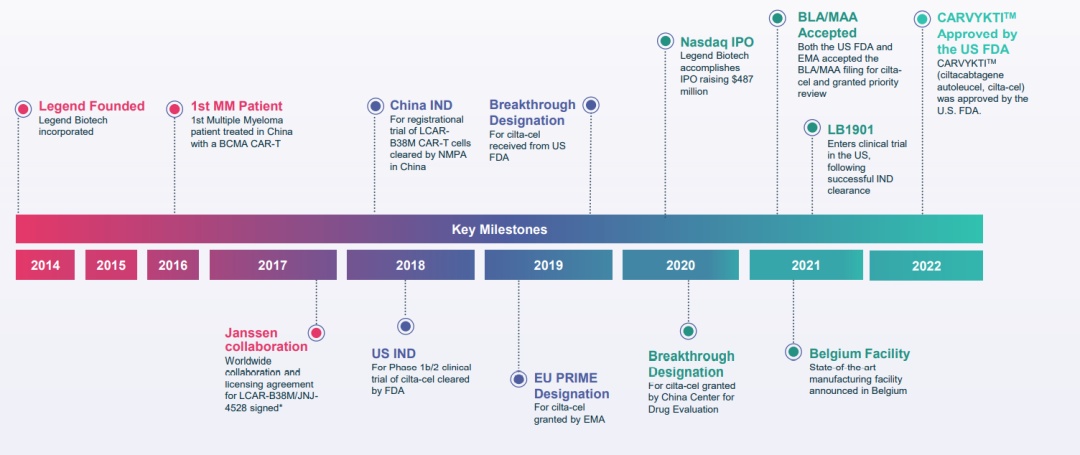
▲ The main milestone of the legendary creature
Year 2014
Legendary creature
2016
In March, China, LCAR-B38M clinical treatment of recurrence, refractory multiple osteoma (RRMM) first patient
2017
In June, the results of the early clinical trials of LCAR-B38M were released at the ASCO conference.
In December, Yang Sen, a subsidiary of Johnson & Johnson, signed a global cooperation and authorization agreement
2018
In March, LCAR-B38M's phase III IND (clinical research application) passed NMPA review
In May, FDA approved JNJ-4528's phase IN IND
2019
In April, the European Medicine Administration (EMA) awarded CILTA-CEL priority drug Prime certification
In December, the US FDA awarded CILTA-CEL breakthrough therapy (BTD, Breakthrough Therapy Designation) certification
2020
In May, it was announced to sign a cooperative research and license agreement with Noile-Immune Biotech
In June, the Nasdaq IPO was listed at $ 487 million, known as "China CAR-T's first share"
In August, Cilta-Cel obtained CDE (Drug Review Center) breakthrough therapy certification
2021
In February, obtained FDA and EMA's biological preparation permit application (BLA)/listing license application (MAA) accelerated review of CILTA-CEL
In June, it was announced to establish a high -level production base in Belgium to expand the global manufacturing capabilities of innovative cell therapy
In September, the IND application approved by the US FDA will begin phase I clinical trials of cell therapy LB1901 for the treatment of adult recurrence/refractory perimeter T cell lymphoma (PTCL), or skin T cell lymphoma (CTCL )
2022
In February, CARTA-CEL, the CAR-T product independently developed by the legendary creature, was approved by FDA to be listed, becoming the first Chinese original CAR-T cell therapy product listed in the United States.
Financing history and equity structure
As the holding subsidiary of the Kingsuri creature, the financing history of the legendary creature is relatively simple, including only 3 milestones, including the IPO, but each financing amount is very good:
In March 2020, it received a strategic investment of $ 150 million. The investors were Hudson Bay Capital, Johnson & Ventuna, Lili Lili Asia Fund, Victoria Capital, and RA Capital. The valuation of the post -investment was $ 1.95 billion.
In June 2020, the Nasdaq IPO was listed and raised $ 487 million.
In May 2021, he received a strategic financing of 500 million US dollars in Gaozhang Capital, Gao Jian entered the Kinshiri creature and the legendary creature owned by Kim Siri.
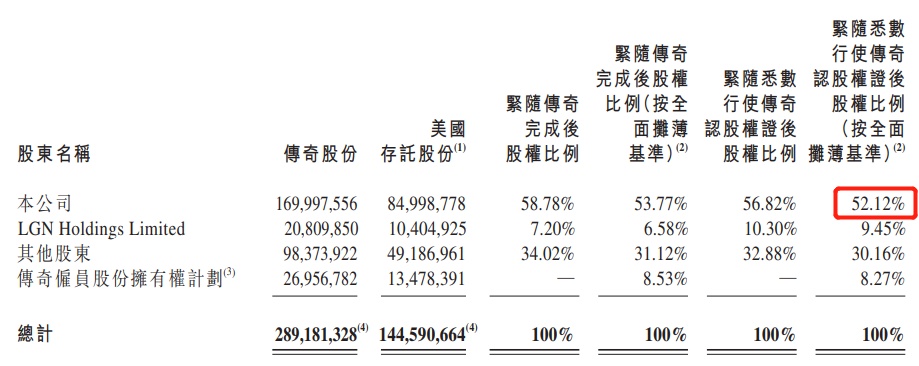
According to the announcement issued by Kingsrui Bio in May 2021, the current Kingsuri Bio holds 52.12%of the equity of legendary biology, which is a legendary biological holding company; and 9.45%of the legendary biological biology shares in Gaozhang Capital.
management team
As of the end of 2021, legendary creatures have 1000+ employees in the United States, China and Europe.
From the perspective of management structure, the structure of legendary creatures is divided into two lines of the United States and China, and Dr. Ying Huang, CEO and Chief Financial Officer, coordinated.
Judging from the background of the executive team, the work experience in multinational pharmaceutical giants is basically standard configuration.
The senior management team of legendary creatures has been turbulent.
In 2018, Dr. Xu Yuan joined the legendary creature as CEO. Before joining the legendary creature, Dr. Xu Yuan served as a senior vice president in Meridas in the United States and was responsible for research and development and commercialization. Under Xu Yuan's in charge, the legendary biological American team expanded rapidly, and the development of CAR-T products has progressed significantly.
In June 2020, legendary creatures were successfully listed in the Nasdaq market in the United States.
In August 2020, Kim Siri announced that Dr. Xu Yuan resigned for personal reasons. Dr. Zhang Fangliang, the founder of the legendary biological holding company, resigned as the president of Kingsuri administrative president and took over as the chief of the legendary biological administration and CEO.

Dr. Zhang Fangliang's experience is quite legendary:
From 1995 to 2002, Dr. Zhang Fangliang served as chief scientist in Xianling Yaa (acquired by Merhado).
In 2002, Dr. Zhang Fangliang and others founded the Kingsley creature. In 2015, it was listed on the main board of the Hong Kong Stock Exchange. At present, the market value of Kingsley creatures is HK $ 56.9 billion. (Market value is June 21, 2022)
In 2014, the Create Create Golden Biological Subsidies -Legendary Biology, expanding the scope of Kingsrui creature. Dr. Zhang Fangliang has served as the chairman of the legendary biological board since May 2015.
Dr. Zhang Fangliang has a doctorate degree in Biochemistry at Duke University.
In September 2020, Dr. Zhang Fangliang was monitored and lived for "smuggling storm". The legendary creature replaced the CEO again and was replaced by the chief financial officer Huang Ying. At the same time, the chairman of the legendary biological board of directors was replaced by Wang Yan.
In November 2020, Zhang Fangliang was arrested for smuggling of goods prohibited by the laws and exports of Chinese law.
In February 2021, Zhang Fangliang was given a bail pending trial by the regulatory agency. As of the announcement of the Kingsuri creature at the time, it was not officially accused.
On May 2, 2022, Kings Rui issued an announcement stating that Dr. Zhang Fangliang, Nanjing Kingsuri Biotechnology Co., Ltd. and three employees and one former employee who had handled the group's import and export activities had been notified by the People's Procuratorate of Zhenjiang City. The investigation has been reviewed, and the procuratorate decided not to file any lawsuits to any unit or individual. Dr. Zhang Fangliang was free and served as the executive director, the risk management committee and the chairman of the Strategic Committee.

Dr. Huang Ying has been the chief financial officer of the legendary biological biology since July 2019, and since September 2020 as the CEO. On December 30, 2021, Dr. Huang Ying joined the legendary biological board as a first -level director.
Before joining the legend,
From August 2014 to July 2019, he served as the managing director and the head of biotechnology stock research in Bank of America. Bo Jian and so on.
Before joining Bank of America, he worked at Wells Fargo (formerly the United Film), Credit Suisse, Gleacher and Barclays.
Earlier, the chief scientist of the Chemical Research Department of Xianlingya (now Merhadon), which focuses on the research and development of small molecular drugs in the treatment fields such as cardiovascular and central nervous systems, is also a common author of many patents and colleagues.
Dr. Huang Ying studied at the Junior Class and Columbia Business School of the University of Science and Technology of China, and obtained a PhD in organic chemistry from Columbia University in the United States.

Sally Wang has served as the chairman of the Legendary Biological Biological Board since November 2020.
Judging from Wang Yan's tenure in the legendary biological holding parent company Kings Rui, she is a marketing "war general".
Since joining Kingsrui in August 2002, he served as a sales customer manager to serve as the current chairman of the legendary biological biology. Officials, presidents and other positions.
At the same time, Wang Yan graduated from Wuhan University and received a bachelor's and master's degree in microbiology. From July 1993-July 2000, Wang Ye also served as environmental supervision engineer of environmental protection monitoring station in Futian District, Shenzhen. After joining Kingsrui, in 2003, Wang Yan obtained a master's degree in computer science from Qiaogang University in the United States, and in 2014, he obtained a master's degree in business administration from senior management staff of China Europe International Business School.
Core technical executive
Legendary creatures have established a global research team composed of more than 370 researchers. Researchers identify potential cell goals and create and evaluate extensive candidate product portfolios.
As "the inventor of China's first major CAR-T technology", Dr. Fan Xiaohu, the co-founder and chief scientist of the legendary biological creature, is the main supporter of its CAR-T product technology.
However, on April 2, 2022, the legendary creature announced that Dr. Fang Guowei was appointed as a senior vice president of the company. He studied and developed global leaders in the early days, and replaced Dr. Fan Xiaohu who left him on March 30. Dr. Fang Guowei will be responsible for guiding the research and development of legendary creatures in China, the United States, and Ireland to promote multiple pipeline products against blood tumors and physical tumors.
Dr. Fang Guowei has rich experience in the field of oncology and immunology::
Before joining the legend, he served as the senior vice president of Zymeworks. He is responsible for the development of new technology platform development and multi -functional biological agents and antibody drugs.
Earlier, the person in charge of R & D in ParamacyCLICS, a company of Albervi, found and developed early development.
Before joining PharmacyCLICS, he worked in Genick and Albervi, mainly engaged in oncology research and discovery.
Dr. Fang Guowei obtained a PhD in biochemistry, cell biology and tumor biology at the University of Colorado, and post -doctoral research at Harvard Medical College from Harvard Medical College. Before entering the pharmaceutical industry, Dr. Fang taught at Stanford University to lead the cancer research project.

Dr. Lida Pacaud joined the legendary creature in January 2021 as the vice president of clinical development of the United States.
Before joining the legendary creature, he served as the head of the global clinical project and executive medical director of the cell and genetic department of Novartis, leading the world's first approved CAR-T therapy-Kymriah's clinical development. Prior to this, Lida worked in Roche and Wyeth. Dr. LIDA PACAUD has a PhD degree and pediatric certification in the National University of Medical University, and has received pediatric tumor and hematology training in France.
Commercial executive
Steve Game has served as the vice president of commercial development in the United States and Europe since July 2018. He has more than 28 years of business experience in biotechnology startups, leading pharmaceutical and medical equipment organizations.

Earlier, GAVEL worked at the new foundation (acquired by BMS) and led the commercial development activities of the BB2121 project of the American CAR-T project.
Before joining the new foundation, he served as the senior marketing director in the Takeda Pharmaceutical and Cancer, leading the marketing strategy and implementation of the best -selling medicine for Takeda Pharmaceutical.
Earlier, he worked as a leadership position of sales, marketing and market access at Syntex, IMMUNEX, Johnson & Johnson and IMS Health.
Gavel obtained a bachelor's degree in finance and business administration in Millersville University in Millersville University in Millersville, Pennsylvania.
Yang Yan has been the vice president of commercial development in Greater China since July 2018.

Earlier, multinational pharmaceutical companies such as Roche, Bayer Tumor, and Novarty Tumor held a variety of sales and marketing leadership positions, and their duties continued to increase. With more than 17 years of business experience, it has extensive professional knowledge in the Chinese market promotion and launching products including GLIVEC, Nexavar, Avastin, and Rituxan.
Yang Chong obtained a master's degree in medicine at Nanjing University, China, and served as a surgeon hospital at the University of China Southeast University.
Product pipeline
The goal of legendary creatures is to promote the application of cell therapy in the field of hematological tumors, physical tumors, and infectious diseases. The product pipeline of the legendary creature is also built.
R & D nature (self -research/introduction)
▲ Legendary biological product pipeline
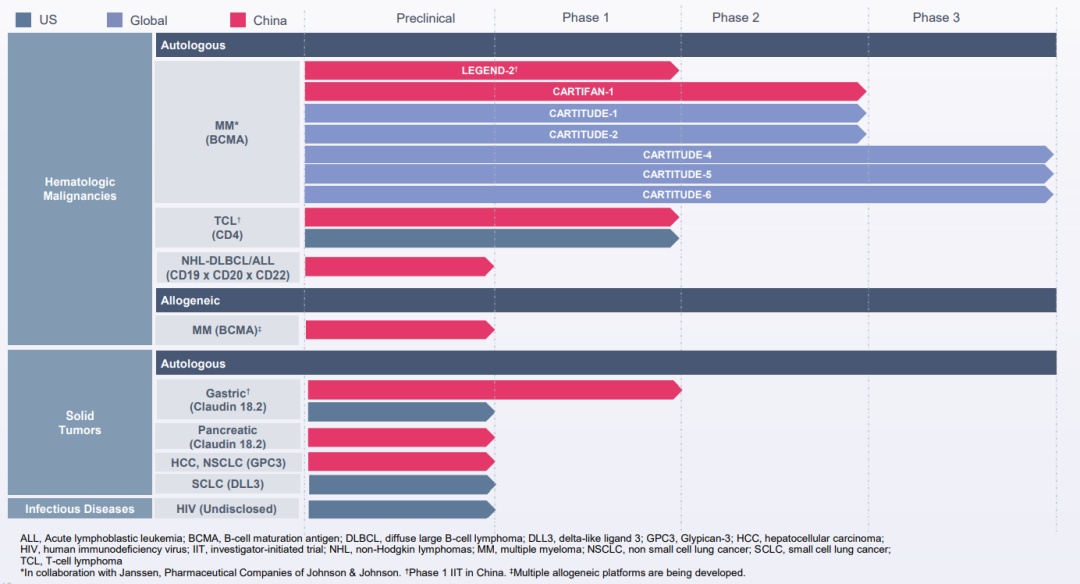
In all the pipelines of the legendary creature, the fastest progress is CARVYKTI (CILTA-CEL) targeting BCMA, which is approved by CAR-T products approved to enter the US market at the end of February 2022.
In addition to Sidaki Olun, there are 8 CAR-T cell therapy products that enter the clinical of the legendary biological pipeline, as well as a project that is not disclosed for infectious diseases.
in,
LB1905 is exploring the dexer car-T therapy;
LB1902, LB1904, and LB1908 are exploring the effects of physical tumors, mainly in the direction of gastric and breast cancer.
product technologies
▲ 4 core technologies of legendary creatures

Legendary creatures currently have 4 core technologies: CAR-T, including general-purpose CAR-T; TCR-T; Car-NK; γΔ-T. All are cutting -edge technologies in cell therapy.
CAR-T cells and TCR-T cells belong to T cells transformed by genetic engineering technology.
CAR-T cell therapy, through identifying membrane surface antigens (such as CD19, BCMA, etc.), it has a significant effect on blood tumors. Many companies have been trying to try to break through the bottleneck of solid tumors. Many CAR-T products have been approved.
TCR-T technology, the main mechanism is to enable the transformed T cells to express the TCR (T Cell Receptor, T cell antigen receptor) of tumor cells, thereby guiding T cells to kill tumor cells. At present, only one product in the world has been approved.
On January 25, 2022, FDA approved Kimmtrak for HLA-A*02: 01 positive irrevised or metastatic uterine melanoma (MUM) adult patients. The product was developed by British biotechnology company Immunocore.
CAR-NK technology, through genetic engineering modification, expressing the chimeric antigen receptor (CAR) that can bind to a specific antigen with tumor specific antigen on the surface of NK cells. The purpose of removing tumor cells. At present, CAR-NK cell therapy is at the clinical stage as soon as possible.
T cells are divided into two categories: αβ T cells (such as CD4, CD8, etc.) and γΔ T cells according to different TCR. Periodontal blood lymphocytes are mainly αβ T cells, and γΔ-T cells generally only account for 1%-5%. γΔ-T cells are natural monitoring cells of the immune system and can identify and target tumor cells. The γΔ-T cells also played the role of bridge closer immune system and the adaptive immune system. γΔ-T cell therapy is currently only clinical.
Product pipeline strategy
Policy and welfare, quickly test candidate products among patients
In order to encourage innovation, the China National Drug Administration (NMPA) launched policy and welfare: After consulting, under the supervision of the Scientific Advisory Committee and the Ethics Committee, Chinese clinicians can launch the clinical test of experimental cell therapy in their hospital. There is no need to obtain the approval of the formal experimental new drugs. Legends cooperate with clinicians and hospitals to conduct experiments in accordance with international standards to support future global regulatory filing and partnerships. This method enables legendary creatures to quickly test legendary candidates in patients, quickly determine the potential candidate products, and promote it to clinical trials registered in China, the United States, Europe, and Japan.
Correspondingly, legendary creatures also adopt a global clinical development strategy.
向 The "frontline application" of similar products to the same type
The effectiveness of the CAR-T product of the legendary creature is very capable.
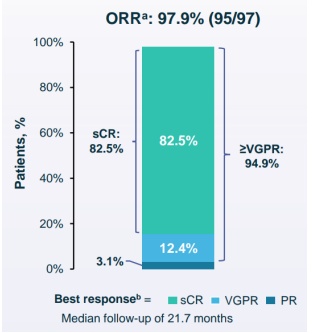
In December 2021, legendary creatures announced the latest clinical data of CILTA-CEL on ASH.
The results of Cartitude-1 clinical trial show that during the mid-range follow-up time of 21.7 months, 97 cases of R/R MM patients treated with Carvykti reached 98%, and the complete relief rate (CR) reached 83. %, 24 months of no progressive survival (PFS) rate reached 61%.
These data once again prove that the clinical trial data of the product is the best. With strong product strength, CILTA-CEL has obtained breakthrough certification/orphan drug certification/priority drug certification in many countries.
With the approval of CILTA-CEL in the United States, legendary creatures will seek CILTA-CEL in the future to promote the front line on the approved indications, and at the same time strive to get more indications.
疗 Explore the "all possibilities" of cell therapy

The legendary creature will promote the development of cytotherapy in other pipelines such as blood tumors, physical tumors, and infectious diseases, and explore more possibilities of cell therapy -have opened different R & D different R & D for blood tumors, physical tumors, and infectious diseases. Planning and related tests.
In September 2021, the legendary creature has begun to develop clinical trials of the 1st phase of cell therapy LB1901.
Ar Introducing differentiated CAR-T production line
In addition to promoting self -developed products, legendary creatures have also been very cautious to cooperate with differentiated technologies.
In May 2020, legendary biological announcement reached a cooperation agreement with NOILE-IMMUNE BIOTECH, an innovative cancer immunotherapy biological company.
Two companies are committed to two specific cancer targets. Legendary creatures have the right to use NOILE-IMMUNE's core Prime (Proliferation Industting and Migration Enhancing, proliferation induction and enhancement migration) technology to develop IL -7 and CCL19 CAR-T and/or TCR-T cell therapy.
The principle of *Prime technology is to transform T cells through genetic engineering technology to express them to express cytokines and trend factor (such as IL-7 and CCL19). IL-7 can maintain the stability of T cells while promoting T cell proliferation, while CCL19 can raise outer T cells and dendritic cells into lymphatic tissue.
At the same time, legendary creatures will pay about $ 70 million to Noile-IMMUNE for partial development, supervision and commercial milestones, and Noile-IMMUNE will also have the right to obtain franchise fees in the commercial sales of products.
This cooperation with differentiated technology companies will enrich its technical route.
Business layout
The creation of CILTA-CEL, the CAR-T product independently developed by the legendary biology, has once again proved that China's innovative medicine has its own place internationally internationally.
This is the first domestic CAR-T product that has been approved in the United States. It is also a key node for legendary creatures to transform from BIOTECH to Bio Pharma after 8 years.
Commercialization
▲ Kings Rui Bio-Legendary Biological Data in 2021
▲ Legendary Biological Data in 2021
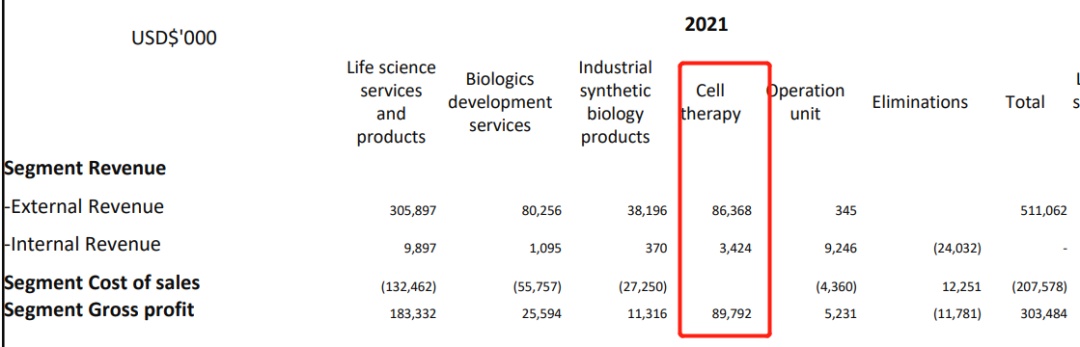
In 2021, the external income of the legendary biology was 86.4 million US dollars, a year -on -year+14.1 % ($ 75.7 million in 2020). The income mainly comes from the continuous confirmation of prepaid and milestones in the cooperation between legendary creatures and Johnson & Johnson.
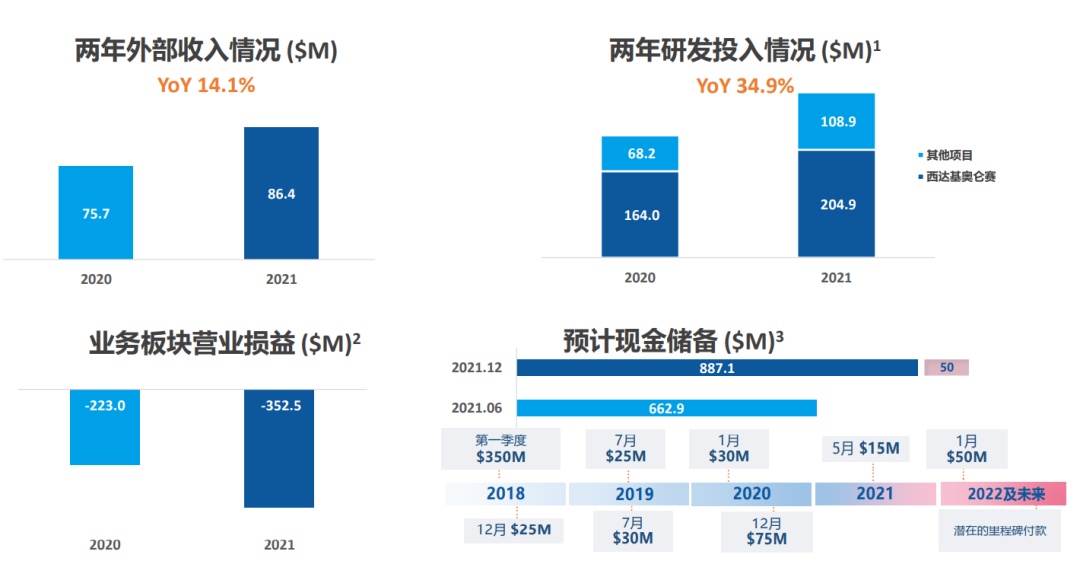
At the same time, because the legendary biological stage is still in the stage of the drug, its net loss is about 390 million US dollars. The adjusted net loss was about $ 350 million.
The main reason for losses is the continuous growth of CILTA-CEL's clinical trials and product R & D costs-the cost of clinical trials in CILTA-CEL clinical trials in the United States and China, about $ 200 million; the research and development costs of other pipeline projects are $ 110 million Essence The total R & D investment was US $ 310 million, a year -on -year+34.9 %.
In 2022, the legendary creature completed the last commercial application of Carvykti products. The commercial team of legendary creatures and Yang Sen will soon begin product sales. Divide. According to Dr. Huang Ying, CEO of Legendary Bio, at the beginning of this year, introduced at the J.P. Morgan Health Forum, Westki Olun is expected to advance to multiple bone marrow early treatment markets in the future, and its expected sales peak will be $ 5 billion.
CARVYKTI is priced at US $ 465,000 in the US market; the CAR-T product ABECMA, which is also targeting BMCA, is also priced at $ 419,500, with revenue of US $ 164 million in 2021.
Regional commercialization
▲ Legendary biological production base
Immediately after the commercialization is approved, production capacity construction will also become one of the key tasks of legendary creatures in the next stage.
Legendary creatures have built GMP-level production workshops that supply clinical trials of CAR-T products in the United States and China.

The commercial GMP production facilities in Laritan, New Jersey, USA have also been put into operation.
Build a future commercial capacity base in Belgium and China. Among them, the Belgian production base was announced in June 2021 and is expected to be officially put into operation in 2023.
Commercial strategy
级 With the power of the world's top pharmaceutical companies
Through the success of "cooperating with international pharmaceutical companies (Johnson & Johnson) to promote the research and development & commercialization" model, it pointed out a relatively feasible path to the sea of Chinese innovative medicines.
At the 2017 U.S. Clinical Oncology Society Annual Meeting (ASCO), legendary creatures demonstrated the research results of CAR-T cell therapy LCAR-B38M for multiple myeloma. The results showed that the objective relief rate of 25 patients participating in the treatment for more than a year reached 96%. Because the research results were too prominent, legendary creatures immediately became the focus of the audience at the time. Attracted the attention of many pharmaceutical companies including Johnson & Johnson.
In December 2017, Johnson & Johnson's Yang Sen Pharmaceutical signed a global strategic cooperation agreement to jointly develop CAR-T cell therapy. Legendary creatures became famous in the First World War.
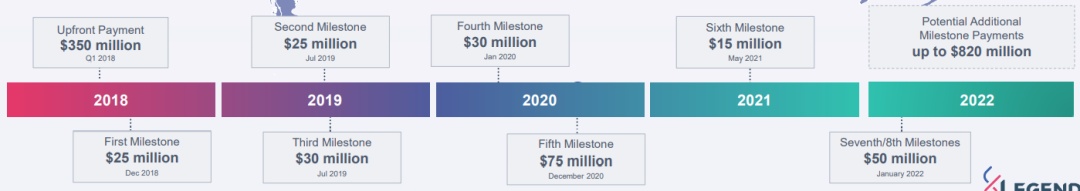
According to the agreement, the legendary creature has obtained a prepaid of $ 350 million, and has the right to get additional payment in the development, production, supervision and sales of milestones. In the global market outside the Greater China, the proportion of legendary creatures and Yang Sen's cost and profit sharing ratio is 50/50, and the amount of the amount in Greater China is 70/30 (legendary creature/Yang Sen).
▲ The process of cooperating with Johnson & Johnson
According to the milestone that has been reached, the prepaid and milestone from Johnson & Johnson has reached $ 600 million. The industry is recognized that Johnson & Johnson's participation has greatly improved the listing of Sidaki Olun.
With the approval of the product, in terms of channels, Johnson & Johnson will "divide and rule" with legendary creatures -legends will participate in the promotion in the United States. Johnson & Johnson will be concentrated in community sales, and legendary creatures will specialize in the promotion of the American hospital market.
储 Pinded cash reserves
The current legendary biological cash reserves are abundant:
At the end of 2020, the cash reserves of legendary creatures were $ 693 million.
At the end of 2021, the cash reserves of legendary creatures were $ 890 million.
In Q1 in 2022, legendary creatures reached two milestones of 50 million US dollars.
The focus of legendary creatures is mainly based on the research and development, production and commercial sales of cell therapy terminal products, and sufficient cash reserves can not only help legendary organisms accelerate pipelines, but also accelerate their commercialization.
层 forward -looking, multi -level layout commercial production centers
For CAR-T products, production is a big problem. This can be seen from the production dilemma faced by the world's first CAR-T product Kymriah (from: Novartis).
Novartis's Kymriah from leukocytes to infusion of CAR-T cells. In clinical trials (Belinda research), patients need 52 days on average; and patients with Geely Yescarta need 29 days.
Data show that 40%of registered patients have never injected Kymriah, and most patients have died of disease progress during the long manufacturing process.
From the production capacity construction mentioned above, it can be seen that legendary creatures have established factories in terms of clinical, early commercialization, and potential expansion of future expansion, which laid the foundation for the commercialization they are going to.
多 According to the region, establish a diversified commercial team
As early as 2018, legendary creatures began to build its commercial team.
In July 2018, Steve Game Vice President of the United States and European Business Development, and Vice President of Commercial Development in Greater China
Yang Chong took office, representing the beginning of the preparation of legendary creatures for commercialization.
With the approval of the product, the legendary creature priced the product at 465,000 US dollars, which was higher than similar listed products (referring to targeting BCMA products). Considering the ability of payment, the pricing of existing products is covered in the United States' commercial and medical insurance.
At the same time, legendary creatures have recruited and built a small and fine commercial team in the past 6 to 9 months. %~ 80%. face the challenge
Legendary creatures are also facing a series of challenges.
Challenge 1: The clinical trial and application ability of the legendary creature itself is questioned
Because Sidako Olun is in cooperation with Johnson & Johnson, the industry has questioned the ability of legendary biology itself to clinical trials and declarations.
The main reason is that the clinical advancement of the domestic market is slow.
In 2018, Sidami Ollen became China's first CAR-T product to enter the clinic. But it has not been approved so far.
In contrast, CAR-T of Fosun Kate and Yaoming Juuo has been approved in China.
The legendary creature explanation was a production base that had no need to meet the GMP standard in China at first, so the company spent some time for the transformation of factory buildings and production that meets the GMP production standards. In addition, due to the influence of the new crown epidemic in 2020, the speed of the patient's entry into the group was delayed. At present, legendary creatures are actively communicating with CDE to complete the group and declaration.
Challenge 2: The succession of the product pipeline
Judging from the current production line, the successor of the legendary biological Sida Kiuron has not yet appeared.
The fastest progress is the CAR-T product targeted by CD4, which enters Phasentery clinical in September 2021.
Challenge 3: Can executives change the blood, can the team remain stable?
As mentioned in the previous executives, shortly after the legendary creature Nasdaq was listed, Dr. Xu Yuan, who had been responsible for the CEO for more than two years, left in August 2020, and then experienced the "storm of Dr. Zhang Fangliang, founder of Kingsley creature. "". Legendary creatures use the chairman of the board of directors and CEOs to calm down the stock price fluctuations.
At the end of February 2022, the legendary creature CAR-T products were approved in the United States. In early April 2022, the "soul figure" of the legendary creature, Fan Xiaohu, co -founder and chief scientist. In an interview after leaving, Dr. Fan Xiaohu said, "I will rest for a while, and then start again, and will still focus on cell therapy in the future." This once again triggered market heated discussions.
Based on CAR-T is one of the areas where Chinese pharmaceutical innovation is most likely to be parallel with the international market, the promotion of CAR-T makes everyone enthusiastically.
But this innovative technology also requires more breakthroughs. The currently recognized breakthrough directions are:
(1) R & D GAR-T to improve the process of CAR-T, so that CAR-T is a "good and affordable" drug.
(2) CAR-T therapy is used for physical tumors to enter a broader market.
(3) Find new targets. At present, the research of CAR-T is basically around CD19 and BCMA. Are there more and better targets that have not been found?
· END ·
- END -
Fuzhou new coronary virus pneumonia epidemic situation
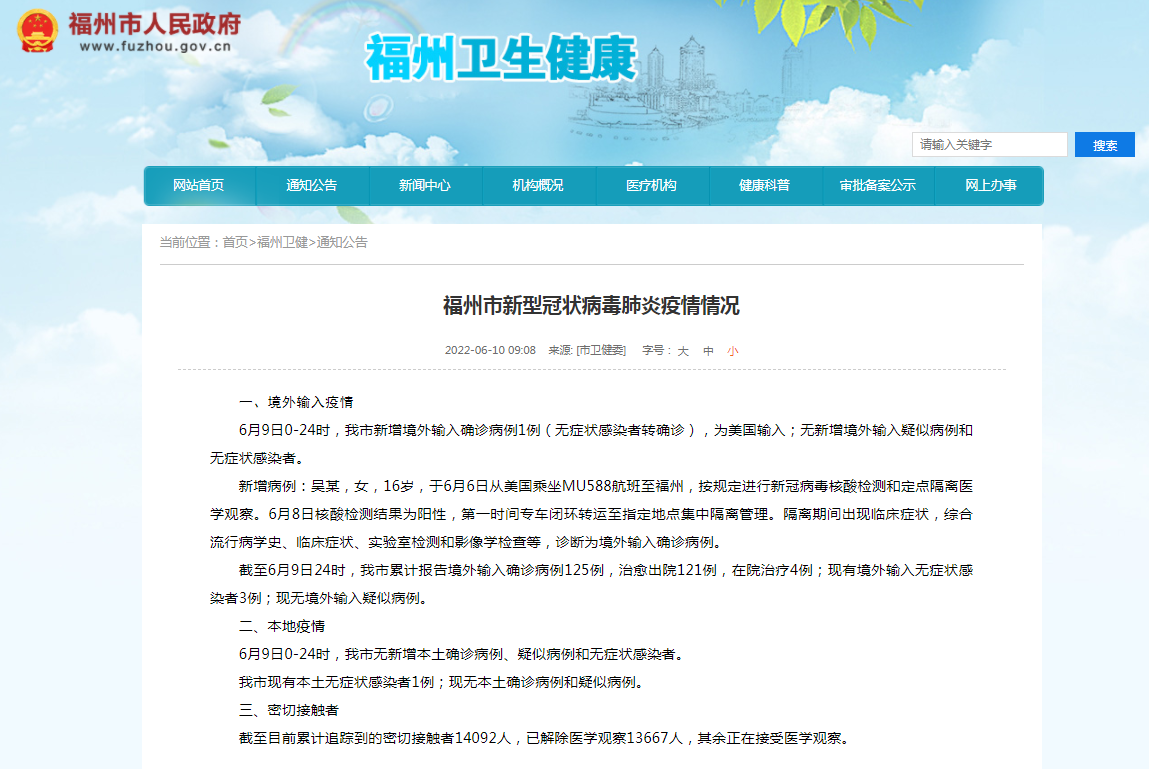
1. Enter an epidemic situation overseas at 0-24 on June 9 Symptoms infecte...
Notice on the collection of illegal crimes such as the production and sales of fake drugs and inferior drugs

Suizhou Market Supervision and Administration Bureau Suizhou Public Security Burea...