The first domestic HPV vaccine is 100% protection rate of 66 months after immunity
Author:First -line Time:2022.09.29
"Liuye Knife-Infectious Disease" recently published the first analysis of the first domestic binary HPV vaccine III clinical trial. Studies have shown that the protection rate of HPV16/18 related lesions within 5.5 years after immunization is as high as 100.0%, and can induce high -level antibodies that last 5.5 years. Experts said that domestic vaccines can provide considerable immunogenicity and protection effect in preventing cervical cancer. Experts suggest that women with age should be vaccinated as soon as possible to prevent the occurrence of cervical cancer.
The first domestic binary HPV vaccine III phase III clinical trial was carried out nationwide in November 2012. The clinical trial was included in 7,372 healthy women aged 18-45 (3689 in vaccination group, and 3,683 in the control group) completed the vaccination and performed for a period of time. 66 months follow -up monitoring. The results showed that the protection effect of vaccination in the primary lesion end in different age groups (PPS) can reach 100.0%; in 6 months of continuous infection, the vaccine aggregate The protection effect was 93.9%and 100.0%, respectively.
In the 66 -month follow -up analysis, the vaccine can effectively induce subjects to produce high and long -term HPV16 and type 18 neutralized antibodies and IgG antibodies, showing good immune persistence. In addition, the vaccine has extremely high safety, and no serious adverse incidents related to vaccination are occurred during the study period, nor the abnormalities of poor pregnancy ending related to vaccine vaccination and abnormal health status of newborn health status.
It is reported that this study was studied by the National Cancer Center/Chinese Academy of Medical Sciences Cancer Hospital, Xiamen University School of Public Health, Jiangsu Province Disease Control and Control Center, Peking University People's Hospital, Liuzhou Disease Control and Control Center, China Food and Drug Institute The team is completed together.
"Domestic vaccines can provide considerable immunogenicity and protection effect in preventing cervical cancer, bringing healthy gospel to women." express.
At present, there are about 200 types of HPV types. According to the carcinogenic rate, it is divided into high -risk and low -risk types. There are more than 20 types of high -risk HPVs. Among them, the highest carcinogenic rate is 16 and 18, about 84.5%of the cervix of cervix. Both squamous carcinoma are caused by type 16 and 18 infections.
Shen Fangrong introduced that cervical cancer is one of the common gynecological malignant tumors and shows an increasingly younger trend. In 2021, ICO/IARC's HPV and related diseases in China show that in 2020, among women 15 to 44 in China, the incidence and mortality rate of cervical cancer ranks third in female tumors.
"Cervical cancer is not terrible, because it is the only cancer that is clear and anti -controllable." Member of the Medical Professional Committee of the Pediatric Branch of the Chinese Medical Association, chief physician, professor, professor, professor, professor, professor, professor, professor, professor, professor, professor, professor, professor, Doctoral supervisor Chen Youguo explained that the first step of the third -level prevention strategy of cervical cancer is the HPV vaccine. The WHO and my country's cervical cancer prevention and control guide at the same time suggested that the HPV vaccine is vaccinated as soon as possible, especially before sexual life. It can play the best protection effect.
In 2020 WHO's "Accelerating Elimination of Cervical Cancer" to the stage of 2030, the first is 90%of the girls in vaccination with HPV vaccines before the age of 15; the second is 70%of women who receive efficiency at the age of 35 and 45. The screen of cervical cancer; the third is 90%of women who have been diagnosed with cervical diseases.
The HPV vaccine is divided into two -valent, four -valent and nine valence according to the virus subtype types covered by the vaccine. Recently, the nine-valent HPV vaccine has been approved by the National Drug Administration of my country, and the suitable population will officially expand to women 9-45 years old. The age of the nine -valent vaccine will make a wave of women lining up to "wait for seedlings".
"When the age of appropriate age does not make an appointment to the nine -valent vaccine, it can be vaccinated as soon as possible or a four -price vaccine. It is not worth it to infect HPV in the process of waiting for high -priced vaccines." Chen Youguo suggested.
- END -
Henan reports the latest epidemic situation!Travel policy changes →

Henan Legal System Henan Legal System Daily was founded in 1984 and was sponsored ...
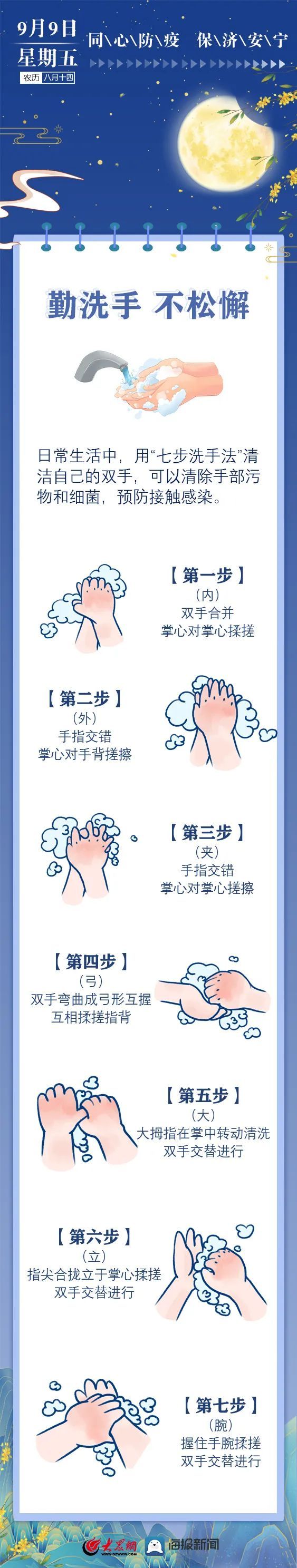
Epidemic calendar 丨 Diligorate toilet to talk about hygiene and do good protection
Concentrate to epidemic prevention, Baoji AnningThe epidemic prevention and contro...