Interview with Li Xiaoyi, chairman of the board of directors of Zhaofe Ophthalmology: The most important thing at the moment is to get the "Admission Voucher" of Atropine. New pharmaceutical companies must survive first
Author:Daily Economic News Time:2022.09.28

■ Related companies: Zhaofe Ophthalmology (HK06622, the stock price of HK $ 2.68, a market value of HK $ 1.452 billion)
■ Core competitiveness: Atropine's eye drops are in Phase III clinical; comprehensive ophthalmology pipelines; advanced production facilities with commercial scale; cooperation with domestic and foreign pharmaceutical companies.
■ Concept: Medicine and Bio; Ophthalmology
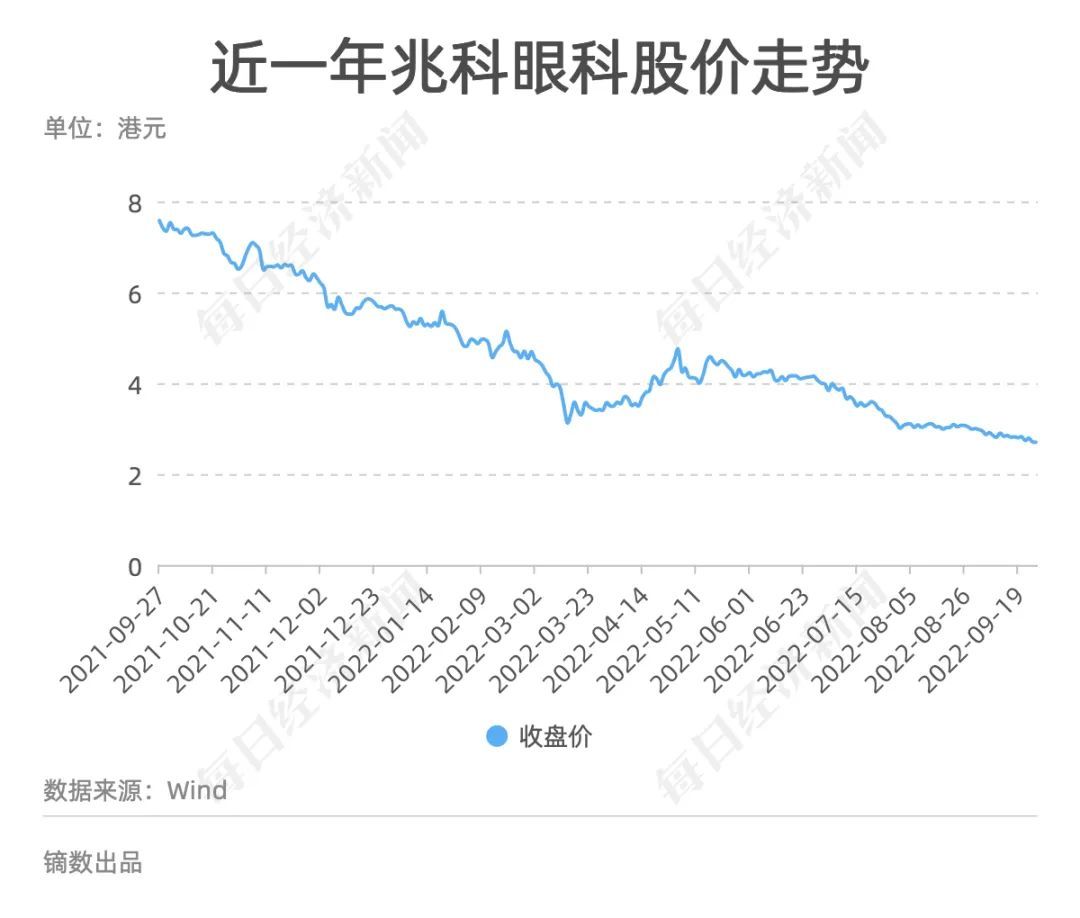
On April 29, 2021, Zhaofe Ophthalmology caught up with the last bus of the Hong Kong Stocks 18A (the "Main Board Listing Rules" of the "Main Board Listing Rules" of the Hong Kong Stock Exchange), which was successfully listed on the market. Zhaofe Ophthalmology is a recruit in the capital market, but Li Xiaoyu, the founder and chairman of the board of directors, is a veteran in the pharmaceutical industry.
As early as 1994, Li Xiaoyu founded the Li Family Pharmaceutical Factory. In 2017, Li Xiaoyu split out the ophthalmology pipeline from the Li's Pharmaceutical Factory, established Zhaobo Ophthalmology, and officially devoted himself to the development of ophthalmology drugs. Right now, Zhake Ophthalmology is used to treat dry ophthalmology, which is declared listing to delay the process of adolescent myopia. Essence
Li Xiaoyu said that ophthalmological drugs are a long -term neglected field. "Because the plate is small, the capital is not concerned." However, the demand for ophthalmology is very large, not only the huge number, but also the demand for new and good medicines is particularly urgent.
According to the World Vision Report released by the WHO in 2020, at least 2.2 billion people around the world suffer from vision damage or blindness. At least 1 billion people's vision injury problems can be prevented or yet to be resolved. Mesopia and dry eye diseases such as myopia and dry eye disease have also been "named" for high incidence.
However, corresponding to the high incidence of eye diseases is that the market space of my country's ophthalmic pharmaceuticals only accounts for only 10%of the entire ophthalmology track, which is in a lower position. The core reason is that the difficulty of R & D, clinical trials lead to a long waiting cycle, and the drug is still highly eliminated after the drug is approved.
Li Xiaoyu said that, including the eye medicine industry, the entire biomedical industry is in the process of pendulum effects, and gradually returns to the middle zone after experiencing overheating and cold. "This is actually a good thing to return to rationality after the big waves of sand and sand. This is a good thing for some companies that really go to research and development and innovation."

Photo source: Zhaofeye Eyee Official Website
Atto eye drops enter the overtime game: "The most important thing is to get the entry ticket first"
The closest and open and transparent competition in the field of ophthalmology should be the racing race of Atropine's eye drops.
This MM choline receptor inhibitors conducted research on Singapore, Hong Kong, China and other places around 2005 to delay the development of adolescent myopia. Earlier, some enterprises used policy to "wipe the balls" to sell the internal preparations in the Atto Hospital in Internet hospitals, but they were called stopping online this year. In this context, who can take the lead in becoming the "regular army" in the field of myopia prevention and control and become a dream of many companies.
Focusing on the track, competition is more intense. Drug clinical trial registration and information publicity platform shows that among local pharmaceutical companies, Xingqi Eye Medicine, Oukang Mito Vision, Zhaoco Ophthalmology, and Hengrui Pharmaceutical have pushed the clinical trials of Atto eye drops to the III phase. Among them, Xingqi Eye Medicine and Zhaobo Ophthalmology Phase III clinical trial shows that the subject has been recruited and is in progress.
No accident, the first name will be born among the two companies. In September of this year, Xingqi Eye Medicine said in exchange with investors that the 2 -year phase III clinical trial has completed the test recruitment and is in the follow -up stage.
Zhaofe Ophthalmology is a "different approach." In 2020, Zhaobo Eye Signed an agreement with NEVAKAR in the United States to introduce the latter's development and commercialization of low -concentrations of eutricate developed in Greater China, South Korea and other regions. In September 2021, the two -year -old III clinical trial (research on children with myopia, also known as "China CHAMP" research) and one -year III Phase Bridge clinical trial (small Champ), which deepened myopia (for myopia, also known as "China Champ". Approved by the State Drug Administration.

Photo source: Zhaoko Eye Half -annual report
In August of this year, Zhaofe Ophthalmology announced that both experiments were completed several months in advance to complete the patient's entry into the group, allowing Zhaoche ophthalmology to occupy the leading position among the developers of Atropine's eye drops.
Today, the racing of Atto's eye drops has entered an overtime phase, but the R & D company dare not let go of the hanging heart. In an exclusive interview with "Daily Economic News" reporter, Li Xiaoyu admitted that "the most important thing at the moment is to get the admission voucher for Atropine's eye drops."
"Daily Economic News" reporter noticed that the "Implementation Regulations of the People's Republic of China (Revised Draft for Revised Draft Consultation)" proposed the concept of "exclusive period", that is, the first new varieties, dosage forms and specifications to be approved for listing. And increasing children's indications or usage dosage will be given a market exclusive period that is not more than 12 months, and the same variety is no longer approved during the period.
This means that whoever can grab the first place has the longest market exclusive period that does not exceed one year. This temptation is obviously huge, but the collision line is not easy. The reporter learned that Atto has not been approved in Mainland China, it has its special reason.
Wang Ningli, the leader of the National Institute of Anti -Blind Technology and the Director of the Ophthalmology Center of Beijing Tongren Hospital affiliated to the Capital Medical University, is also a leading researcher at the Zhake Ophthalmology NVK002 III Phase III Clinical Test. Wang Ningli said in an interview with the "Daily Economic News" reporter that in Western countries, myopia is not a clinical needs that need to be resolved, so they have not bet on such drugs too much resources and energy. However, Asian adolescents have high myopia, especially the adolescents' myopia rates in our country are high and they are still growing. Therefore, we need to approve the listing of Atropine's eye drops before the mature European and American drugs. Essence Wang Ningli continues to explain that children and adolescents are particularly serious. The clinical trial design period for Aduradinial eye drops in my country is 3 years. Clinical trials can observe the safety and effectiveness within 3 years. What are the safety and effectiveness of the drug after a large -scale and long -term application? This needs to be observed.
"Attoppin eye drops are approved, we need not only the data and results of the drug registration test, but also the longer observation period after listing," Wang Ningli said.
It can be seen that whether it is a drug supervision agency or the top expert in the industry, you have a cautious attitude towards Atto. Atropine's eye drops want to get the approval, and continue to wait. For R & D enterprises, the listing of "seconds must fight in seconds". In the interview, Li Xiaoyu lamented, "As long as Atropine's eye drops are listed, no matter how long observation periods will be after listing, they will be willing."
There is another option for Zhaoyo Ophthalmology. That is, Atropine's overseas partners are conducting Phase III clinical overseas. Once they are first approved overseas and then introduced to domestic applications to apply for registration, it is also a way. "This road may be faster." Wang Ningli said.
The ophthalmology track is not afraid of "inner rolls"? There is still a huge mismatch between the supply and demand of ophthalmology
Compared to the urgency on Atropine, the Zhaoche Ophioplasses can be temporarily relieved on the dry eye cinger A eye gel.
In June of this year, Zhaofe Ophthalmology announced that the application of cyclopylacin A eye gel was applied for the acceptance center of the State Drug Administration. Circin A -eye gel enters the countdown period of listing, and this drug will also become the first commercialized independent development and innovative drugs for Zhake Ophthalmology.
Demonstration of dry eye disease is not inferior to myopia prevention and control. According to the Ferrisana report, it is expected that the domestic medium -weight dry eye disease market will increase from US $ 300 million in 2024 to US $ 1.6 billion in 2030, with a compound growth rate of 34.1%. As a "star" drug for dry eye, Eljian's Restasis annual sales have exceeded $ 1 billion.
The company's innovative medicine list. Photo source: Zhaofeye Eyee Official Website
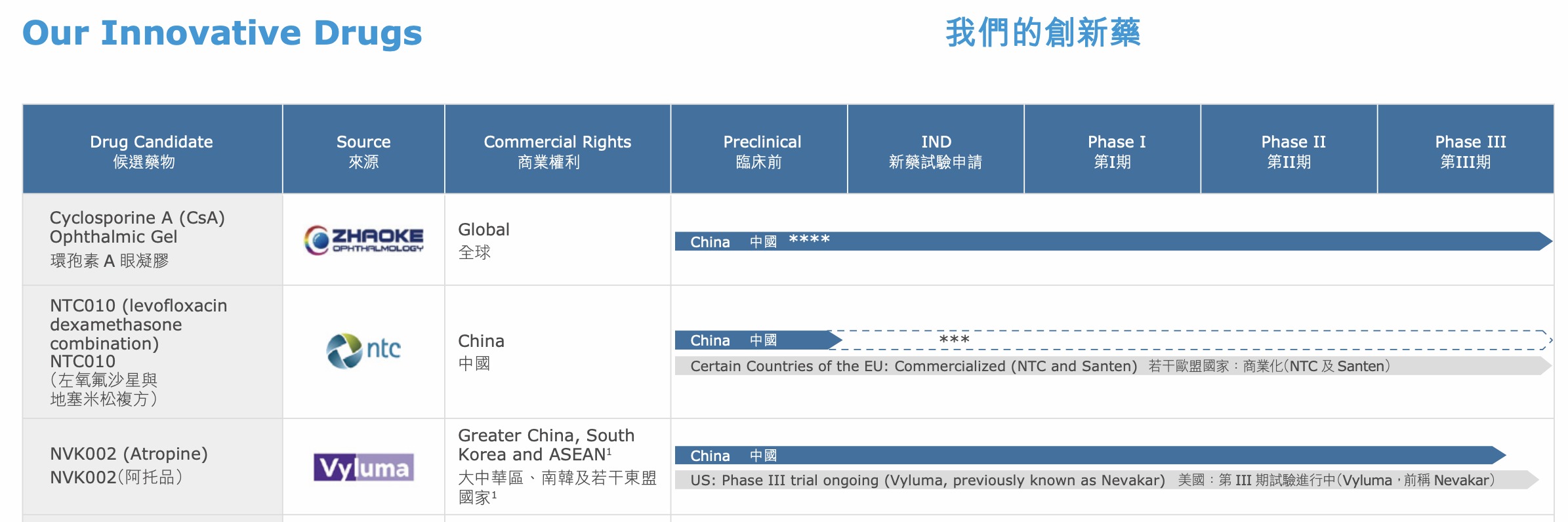
In China, the first imitation drugs of RESTASIS have been listed and included in medical insurance.
Li Xiaoyu believes that compared with the cyclosporine products on the market, the cyclosporine A eye gel in Zhaoche has differentiated advantages. Its gel dosage forms are faster, and they have the advantages of only once a day, which is better in compliance.
More competitors also target the dry eye market. On March 29 this year, Hengrui Pharmaceutical announced that the SHR8028 drop of eye fluid added a new III clinical trial application to obtain NMPA (State Drug Administration) approval. SHR8028 is a dry eye drug introduced by Hengrui Medicine in 2019. At the same time, more generic drugs have been pushed to the market.
This brings a hidden concern. The diseases in front of the eye include three major indications -myopia, dry eye disease, and old flowers. The competition has been in full swing. How can the eye medicine research and development enterprise avoid falling into the "inner roll" predicament?
In this regard, Li Xiaoyu told reporters that there are still huge mismatches between China's ophthalmological drug supply and demand. He said, "Take dry eye drugs as an example. Before domestic drugs were listed, dry eye patients could only use artificial tears to relieve. From the perspective of market data, the market size of artificial tears was very huge. After that, quickly entering the medical insurance, it shows that there are huge unsatisfied clinical needs of dry eye. Therefore, whether it is a few drugs, the market demand is there, not only the scale and urgency. "
Wang Ningli also believes that although there are five or six drugs in Atropine's eye drops, the phenomenon of "tie" must exist, but at the same time, it is necessary to see that diseases such as myopia have risen to public health problems. 50%, the scale of about 700 million people. Therefore, the market is huge, and ophthalmology companies should not be afraid of "inner rolls".
Although the market is broad, as the thinking of other new drugs, eye medicines must be differentiated. Wang Ningli mentioned that taking Atropine's eye drops as an example, his early research papers have already been published, and the second different concentration cannot be used as a patent. Therefore, it can only be differentiated from the aspects of reducing side effects, comfort, dosage forms and production processes. It is understood that Zhake Ophtaid's Atropine Drops can solve the instability of low -concentration Atropine, and does not contain preservatives. It is expected to have more than 24 months. It has innovative improvements at the level of side effects and convenience.
In terms of existing drugs, my country's eye medicine R & D companies are also promising.
Another department of drug representatives stated in an interview with the "Daily Economic News" reporter that the bottom injection method of eye drugs usually requires a large medical burden on patients and medical institutions. Compliance, security, etc. have brought tests.
Li Xiaoyu also mentioned this. The overall penetration rate of bottom -up drugs in China is very low because of the huge treatment burden brought by existing drugs. "How to solve this problem, how to innovate and break through is that domestic eye drug companies need to be deep." According to Li Xiaoyu, in recent years, many important breakthroughs in the ophthalmology field are concentrated in gene therapy. He believes that this innovative therapy will also show his skills in the next ten years. "On the one hand, many genetic ophthalmological diseases are related to sensitivity cells. Gene therapy can introduce healthy genes to the bottom of the eye, thereby directly treating the disease. On the other hand, the surrounding environment of the eyes is relatively closed, and immune to foreign materials will be immune. Reaction, this 'immune exemption' is particularly conducive to gene therapy. "
This year, Wang Ningli also publicly mentioned that "the current current" National Basic Drug Catalog (2018 Edition) "can no longer meet the current eye disease status and meet the needs of clinical diagnosis and treatment. It is recommended The demand for changes in Chinese ophthalmology, better curative effect, and more clinical value of ophthalmology drugs and appropriately transferred the discharge of ophthalmologists with less clinical use and large side effects. "
In general, the demand for domestic eye medicines is huge, and the demand for new drugs and good medicines is more vigorous. How domestic R & D companies follow up to meet the current clinical needs is the opportunity of eye drug companies.
Photo source: Photo Network-300285662
Now is the golden age of ophthalmology: "The most important task is to survive first, it is cash flow"

In 2017, Li Xiaoyu, who was nearly sixty years old, split out the ophthalmology pipeline from the Li's Pharmaceutical Factory and established a subsidiary specially engaged in ophthalmology development, Zhaoco Ophthalmology. For five years in the development of ophthalmology drugs, Li Xiaoyu said that it is now the "golden period" of ophthalmology drugs.
"In the past, ophthalmology drugs were not very valued. Traditional pharmaceutical companies believed that this field was too small. In the past, myopia was resolved by laser surgery. No one thought of using drugs to prevent and control. When the plate was small, the capital also felt that it was not attractive." Li Xiaoyi said Essence
Long -term ignoring or insufficient attention, so that domestic eye medicine research and development still has a long way to go. Right now, domestic ophthalmology drugs are still dominated by multinational enterprises, and the number of innovative pharmaceutical companies focusing on ophthalmology is still small; innovative therapy represented by gene therapy has been developed in the field of ophthalmology. Essence
According to the data of the "2022 Ophthalmology Industry Research Report", the imported drugs accounted for more than 50%of the domestic key provinces and municipal public hospital ophthalmology medicines. The top 4 of the ophthalmological drug market are the 25.35%of Swiss Novartis, 14.24%of Japan, and 6.32%of the German URSAPHARM, and Shenyang Xingqi Eye medicine (SZ300573, the stock price of 87.28 yuan, the share price, which is 87.28 yuan. The market value of 7.689 billion yuan). Domestic ophthalmology companies have only one seat.
Chasing the motivation that is inseparable from continuous. In addition to external capital, the power is more important to rely on itself. Since the beginning of this year, a number of new drug companies have "developed, injured on one side", faced with ebb capital and stretched economic strength, some people chose to stop the project that is still far away, and some people tighten their expenses to wrap tight cotton coats for winter.
Li Xiaoyu, who has been in the pharmaceutical industry and the capital market for many years, admits that he has been used to this trend. "What comes fast is destined to go fast. The pharmaceutical industry is never a short and fast industry. This industry has high, low, and cycle. "Horse horses."
Li Xiaoyu admits that the advantage of listed companies can be seen by capital, but capital support will not be endless. And the capital perspective of different markets is different. For example, the US capital market will see a good clinical data or nodes that complete different clinical trials, and give rewards. But in China, more importantly is to see the results and see how this product sells.
"For an enterprise, the most important task is to survive first, and there are cash flow as soon as possible. So we must have a product to let everyone see it as soon as possible, so as to give ourselves for time. More patience, more that companies must ensure that they can withstand some failures. "
According to the financial data of Zhake Ophthalmology, since the listing of April last year, with the IPO raised funds, the company currently has more than 2 billion yuan in cash reserves to support product research and development in the next 2-3 years. In the first half of this year, the company's R & D expenditure was 101 million yuan. The company predicts that in addition to the clinical clinical clinical of the project to increase the investment in R & D investment, sales and marketing expenditures will be increased in 2022 to support the landing of product commercialization.
Photo source: Zhaoko Eye Half -annual report
Zhake's eye medicine, which has no product listing, is waiting for the commercialization of the product to "return blood" to give yourself more attempt. Li Xiaoyu said that Zhake's ophthalmology hopes to realize his own hematopoietic cycle through his own commercialization, so that the company can run it by itself.
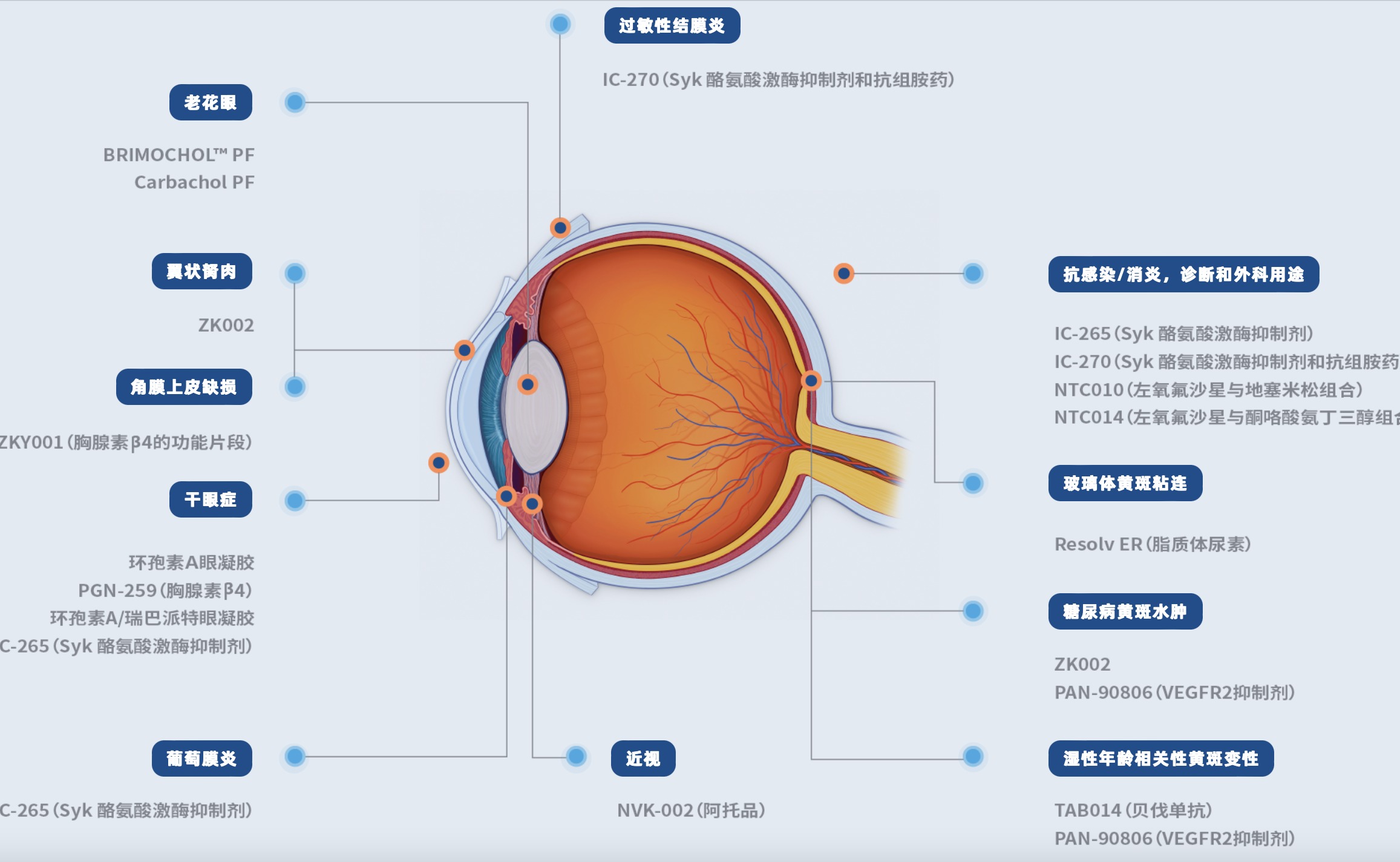
"Many things are pendulum effects, and finally stay in the middle. This is actually a good thing to return to rationality after the big waves and sand. This is a good thing for some companies that really go to research and development and innovation. It is also very large. It is not a good thing to do a more solid thing to seek speed. "
Li Xiaoyu finally said, "In the process of repeated pendulum, everyone should understand the characteristics of the pharmaceutical industry more clearly, so that the industry can develop healthily. If the outside world has unrealistic expectations for us, then it will soon be disappointed, and the industry will lose the industry.With support, this is actually a vicious cycle. "Reporter | Chen Xing
Edit | Yang Xia
Vision | Zou Li
Capture | Yang Xia
Daily Economic News
- END -
Xinhua Quan Media+| She treats patients as friends, and the patient treats her as a family -Zheng Guangmin, "Baihu En -style Doctor" in Lanzhou, Gansu

August 19th is Chinese Physician's Day.Zheng Guangmin is the director of the Depar...
Most of the History of History of History History and History of Multi -Men was infected with Xining
Xinhua News Agency, Xining, June 13 (Reporters Liu Zexing and Li Ning) The Chengdong District People's Court of Xining City, Qinghai Province, opened the trial of the defendant Ma, Yemou, and Moumou i...