New hopes for patients with gradual frost, a kind of innovative drug is expected to reverse the progress of the disease
Author:Medical community Time:2022.09.27
"What we lack is not hope, but the time for hopes."
Written article | Ling Jun
Source | "Medical Community" public account
Bleak
On September 22, 2022, a 3 -phase clinical trial results released by the New England Medical Magazine showed that an innovative genetic drug Tofersen slowed down and even reversed the course of the course of the disease of some patients with galaxy.
"I can walk away from the cane and stop the use of some painkillers." The 68 -year -old Les Wood is the first participant in the test. So far he has been suffering from gradient for 10 years.

Les Wood will walk alone at home after treatment
Gradient, that is, muscle atrophic spinal cord side cable sclerosis (ALS), is widely known for physicist Stephen Hawking. Hawking was diagnosed at the age of 21 until he died for 55 years with the illness. But he is a special case. The average adult survival of patients with fragrant disease is only 3 to 4 years, and less than 10%can survive for 10 years.
After the diagnosis was diagnosed in 2012, LES Wood lost his ability to work, and his nurses and wife who gave up his career was also his career. As a degenerative neurological disease, the whole body muscles suffering from felmeal will gradually shrink and lose their ability to move. In the later period, it will cause difficulty swallowing, until respiratory failure, and death.
A person who was originally healthy seemed to be frozen into ice cubes by time. Some statistics show that there are about 500,000 gradual freezing patients worldwide.

Stephen Hawking
At present, the cause and pathogenesis of galaxy disease are not clear, but about 2%of patients are considered to be mutated by SOD1 genes, causing nerve damage caused by toxic protein. And Les Wood is these 2%.
In 2016, as the first batch of patients, Les Wood participated in the clinical trial of Tofersen in a wheelchair. Researchers recruited a total of 108 patients with mutations in SOD1 gene mutations. The drug group had to perform lumbar spine puncture once a month. The needle passed between the bones of the spine and injected the drug directly into the spinal fluid.
The first 6 -month test results were not ideal. There are no significant clinical differences in the relevant disease scores, activity ability and lung function in the drug group and placebo.
However, the test also found that compared with the placebo group, the total concentration of SOD1 protein in the cerebrospinal fluid of the pharmaceutical group was significantly reduced. Based on this promising sign, the researchers have extended the administration cycle -63 patients with drug groups continued to take the medicine until 12 months, and the other 32 comfort agent groups began to use the medicine from scratch.
In the end, it was found that for patients with "slow disease progress" before treatment, muscle strength improved after one year of treatment, and the severity of the overall disease remained stable. For patients with "heavy diseases", the speed of muscle function decreases significantly.
At the same time, the sooner the medicine is used, the more obvious the effect of treatment. Except for Les Wood, another patient who received treatment said that he had been able to write a greeting card again.
Compared with the negative test results of 6 months, researchers analyzed the time after 1 year, "lagging only reflects the time required for the healing of motor neurons and the difference in clinical manifestations."
Incadence efficacy and the "life -saving straw" of the patient
In the past 40 years of research, only two drugs around the world have been approved by the US FDA for treatment of gradients, but they cannot cure them and can only delay the process of galaxy disease to a very limited extent. One of the drugs, related data shows that it may only delay the survival period of 2-3 months.
Earlier, another new drug treatment of AMX0035 has caused heated discussions. The clinical trials of the 137 -person scale show:
Safety is good, and it can still show certain survival benefits for about 3 years. Compared with the placebo, it reduces the risk of death by about 44%. But during the 6 -month random period, AMX0035 did not find a significant difference in survival in statistics.
However, in March of this year, the US FDA Weekly and Central nervous system drug consulting committee concluded that this study failed to provide "sufficient evidence", including too small test scale and lack of data, etc., which initially rejected the medicine.
The industry analysis may be because of the admission to ADuhelm's front car -ADuhelm was approved by the US FDA for Alzheimer's treatment on June 7, 2021, but the FDA was questioned due to the lack of credible efficacy data.
The patient's attitude is the opposite of experts. After FDA's negative attitude towards AMX0035, according to overseas media reports, thousands of emails were sent to the FDA Commissioner's Office, and they requested FDA to re -consider the approval of the drug. This month, the same committee held a meeting again, and this time it was recommended to approve AMX0035.
Similarly, compared to most of the heavy innovative drugs in other diseases, Tofersen's role is very limited. Dr. López de Munain, the Spanish Institute of Neurological Defense Diseases, said, "Improve biomarkers is one thing, and restore or restore or may It is another matter to prevent the disease. "
To some extent, the root cause of Tofersen's concern is that patients with galaxy disease tried to grasp all hopeful "life -saving straws". No matter what the result, they had no choice.
Prevent some gradually frozen disease from the source
This Tofersen is an antonymine oligonucleotide (ASO) drug. As one of the representatives of small nucleic acid drugs, the mechanism of ASO drugs, in principle, understands that through the principle of alkaline base matching, combined with target MRNA, inhibit gene expression to prevent "bad" protein production. Its advantage is that as long as the pathogenic gene is found, the corresponding drugs can be designed targeted, and the disease is inhibited from the source.
In addition to TOFERSEN, in a study published in "Nature · Medicine" in December 2021, scientists also designed another ASO therapy for felmeal. The function score and other indicators of a patient after treatment are basically stable or slightly improved.
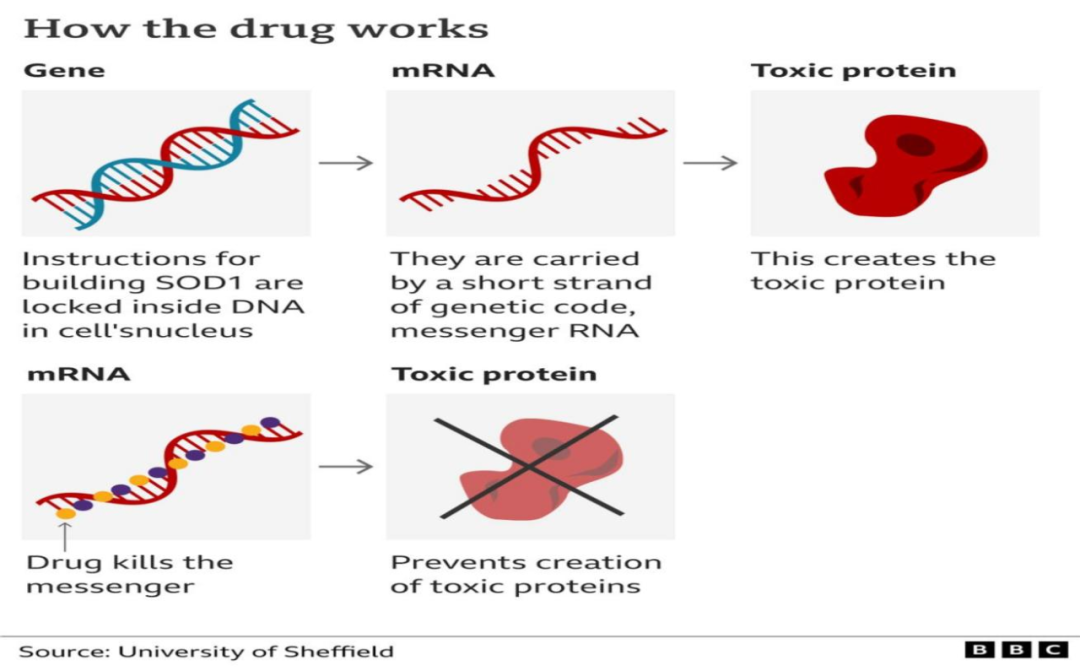
But for gradients, the shortcomings of related therapies are also very obvious, first of all, the limitations of "target gene".
Among global freezing patients, a data shows that only about 20%of cases may be related to genetic and genetic defects. For this TOFERSEN, it is only applicable to patients with sod1 gene mutations -about 2%.
On the other hand, although Tofersen is called "a milestone breakthrough" in delaying the course of delaying gradient disease, it still cannot achieve completely cure. In the early stage of the disease, this drug prevented further nerve damage and did not produce new motor nerve elements. Part of the damaged neurons may take one year to recover and form a new connection with muscle tissue.
In addition, the results of clinical trials emphasize that Tofersen should be used early, but its longer efficacy and security data still needs to be verified.
However, the co -author of the clinical study, Dame Pamela Shaw, a professor of neurology at the University of Sheffield, believes that what makes the frosty disease so terrible is the speed of change. If it can stabilize the disease to the greatest extent, it is a huge achievement. At the same time, if the drug is eventually proven to be successful, it will also help accelerate the research progress of genetic drugs similar to genetic drugs, and establish a breakthrough for the treatment of gradients.
It can be seen that from traditional chemicals and protein drugs to today's fiery gene/cell therapy drugs, more and more "terminal illness" may be cured. Recently, scientists have also developed a gradient therapy based on genetic engineering combined with stem cell therapy. The results of the phase 1/2A clinical trials were published on September 5th in "Nature · Medicine".
But as a rare patient family member previously told the "medical community", "what we lack is not hope, but the time waiting for hope." For patients with gradients, only the average survival period that they wait, only there is only the only period of survival. Less than 5 years.
It is reported that the US FDA has accepted the Tofersn listing application in July this year and included in the priority review qualification. It is expected to make a decision on January 25 next year.
references:
[1] 'TRULY Remarkable'drug Helps Motor Nerone Disease, https://www.bbc.com/news/health-62851186
[2] FDA Seems Poised to Approve a New Drug for ALS, But Does It Work?, Https://www.gpb.org/news/shots-health-news/2022/09/fda- seems-poised- APPROVE-New-DRUG-FOR-ALS-IT-Work
[3] BIOGEN TRUMPETS DATA FROM ALS TRIAL As Fda Decision Looms, https://pharmaphorum.com/news/bioGen-data-from-s-fda--Decision-Looms/
[4] Experimental Shows Signs of Slowing Motor Neurone Disease, https://www.theguardian.com/society/2022/sep/ExperIMENTAL-SLOWING-MOTOR-EuRONE-SEANERONE-SITOR-SLOTOR-Motor-Eurone-SEURONE-SEURONE-SEURONE-SEURONE-SEURONE-SEURONE -DINERONE -DININEE
[5] FDA Community, in Reversal, Favors Amx0035 APPROVAL for ALS, https: //ALSNEWSTODAY.com/news/fda--Reversal- AMX0035- APPROVAL-ALS/
[6] How far is the spring of frozen people? Blood and honey behind albrioza, https://baijiahao.baidu.com/s? ID = 1738010653107880166wfr = spiderFor = PC
Source: Medical Community
School pair: Zang Hengjia
Responsible editor: Tian Dongliang
*The medical community strives to make any promises and guarantees for the accuracy and reliability of the contents of the content when passing the review, but does not assume the timelyness and integrity of the quotable information (if.) Any responsibility caused by the outdated content and the may not be accurate or incomplete in the cited information. Relevant parties are requested to check or use this as a basis for decision -making.


- END -
Itchy scalp, itchy throat, itching on your body ... The doctor teaches you to deal with all kinds of itch!
Itching appears, not only physical discomfort, but also affects emotions. Share some simple and practical coping methods, try it.Itchy ears: Don't use a cotton swabDing Huizhen, chief physician of the...
Patients produce antibodies due to long -term infusion of platelets, and the staff is "precise blood transfusion" through genetic modeling

Wuhan Evening News, June 27th News The 80 -year -old recycled anemia Patients with...