Lessons from PI3K inhibitors: High ORR data may be just "short checks"
Author:Yaizhi.com Time:2022.09.27
Lessons from PI3K inhibitors: High ORR data may be just "short checks"
Source: amino observation/Fang Taozhi
The objective relief rate (ORR) has always been one of the clinical replacement end points for anti -tumor drugs.
Objectively relieving refers to "the sum of the diameter of the tumor lesion is a very high level than the baseline level." Usually, malignant tumors will not be automatically shrunk automatically without treatment. The higher the objective relief rate, it means that patients have a positive response to treatment.
Many tumor drugs at home and abroad have obtained tickets for listing to take the lead in relying on very high objective relief rates.
However, although the objective relief rate can reflect the effect of the drug to a certain extent, the gold standard for evaluating the efficacy is still the total survival period (OS).
The objective relief rate data is not completely equal to the benefits of the overall survival.
PI3K inhibitor is the best case. Once upon a time, with the explosive data of an objective relief rate, a PI3K inhibitor obtained FDA to accelerate the listing.
But unfortunately, some PI3K inhibitors do not extend the survival of patients.
On September 23, the Cancer Drug Consultation Meeting held by the FDA was therefore against the overwhelming voting data to oppose the PI3K inhibitors Duvelisib for recurring or refractory chronic lymphocytic leukemia (CLL) or small lymphocyte lymphoma (SLL). Essence
In fact, the attitude of the FDA for PI3K inhibitors has been tightening since this year. First, the PI3K inhibitors rely on the clinical listing of one -arm, and then deny the value value of the indication of the PI3K inhibitor that has been listed on the market.
When the leaves fall, the autumn, a PI3K target is behind the return, reflects the contradiction between the objective relief rate and the overall survival period.
If you put aside the overall survival period, it is not completely reliable to talk about the anti -tumor effect of the drug with an objective relief rate.
/ 01 /
The mature target of the thirty years of background shows the amazing and objective relief rate
PI3K inhibitors may be unexpected this year.
To say that PI3K (phospholipidalol 3-kinase) is a mature target in the field of tumor therapy, no one will have objections. After all, PI3K has discovered for decades, and as early as 1988, scientists discovered the PI3K signaling pathway.
In further research, scientists have found that the PI3K-AKT-MTOR signaling pathway can control multiple cell processes, including metabolism, exercise, proliferation, growth and survival, but its abnormal activation will also provide convenience for cancer cells. It is human cancer. One of the most common carcinogens.
The mechanism of the signaling pathway regulated by PI3K has been clear. Therefore, cutting off this signal pathway to control the occurrence of cancer has also become an ideal cancer treatment target.
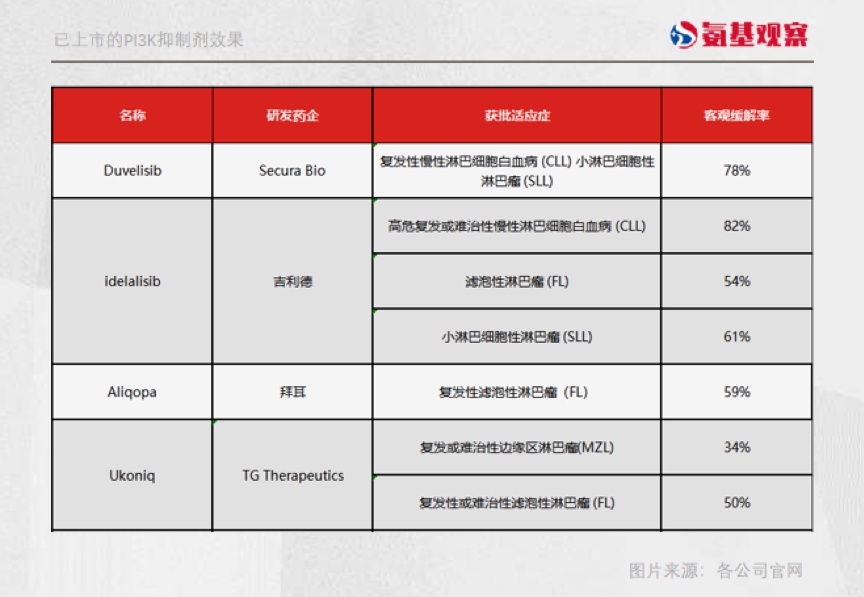
Afterwards, PI3K inhibitors have also been fully verified. So far, 5 PI3K inhibitors in the world have been approved for listing. Based on the results of these approved production batches, the objective relief rate data was shocked.
In 2014, Geely's PI3K inhibitor Ideelalisib and placebo were combined with Merohua, respectively to treat recurrent chronic lymphocytic leukemia (R-CLL) clinical results:
The ORR (objective relief rate) of the IDLALISIB group is 81%, and the placebo group is only 13%, which means that eight of the ten patients can cause tumor reduction, and only one patient in the placebo group responds.
Based on this excellent performance, Ideelalisib was the first to be listed and became the world's first PI3K inhibitor.
As shown in the figure below, the performance of several other PI3K inhibitors in the objective relief rate and no progressive survival period can also be called dazzling. In the indications of Duvelisib's approval, the objective relief rate is as high as 78%.
Therefore, at that time, FDA approved them for listing with these data.
The PI3K inhibitor has also attracted many domestic players to enter the game. The fastest progress is the Stone Medicine Group. The PI3K inhibitor introduced in March this year has been approved to be listed, which is Duvelisib.
In addition, PI3K inhibitors of domestic strength players such as Hengrui Pharmaceutical, Cinda Bio, and Junshi Biological are also on their way. Such a large pharmaceutical company is investing in drug research and development of this target, which also reflects the attractiveness of PI3K inhibitors from the side.
However, even if the mechanism is clear, the proprietary pharmaceutical nature is confirmed, and the blessing of the endemic halo of the large pharmaceutical company, the PI3K inhibitor is still flipped.
/ 02 /
Can't extend the OS, the target of "ashore" also flipped up
The recent turning record of PI3K inhibitors occurred three days ago.
On September 23, the FDA held a tumor drug consultation meeting. The purpose was to treat the PI3K inhibitor Duvelisib three -line treatment of chronic lymphocyte leukemia (CLL)/small lymphocyte lymphoma (SLL) indications for the PI3K inhibitor. Choose.
So, why is a drug that has been approved to be listed, why is it again tried by the FDA?
This is because this drug was approved based on the accelerated approval of clinical replacement end PFS and ORR.
The so -called accelerated approval is a fast channel provided by the FDA to the new drugs for treating serious diseases, so that these new drugs can rely on clinical replacement end to declare and go public.
However, these drugs will continue to be clinical to prove the efficacy. If OS data is not as expected, the indications will still be withdrawn. Today, Duvelisib has encountered a dilemma of OS data. In clinical trials called Duo, the median survival period for patients treated with Duvelisib was 52.3 months, and the median survival period for patients treated with ofatumumab was 63.3 months.
The survival benefits of the treatment group are not as good as the control group, which is obviously a bit down.
Seeing this, you may feel puzzled, why is the PI3K inhibitor's objective relief rate is super high, but it has not been able to extend the patient's survival?
The answer may be that the firepower is too fierce.
Because PI3K exists in cells, if you want to achieve the effect of inhibiting tumors, you need to increase the dose of drugs. But at the same time, large doses of PI3K cutting one -size -fits -all will inevitably affect the normal effect of other cells and bring serious side effects.
It can be seen that the incidence of bad events in the Duvelisib group is as high as 15%, while ofatumumab is only 3%. In other words, Duvelisib used to use the tricks of killing the enemy a hundred self -damage, and the tumor disappeared the patient's body.
Obviously, this is not the characteristics of an excellent anti -tumor drug, and the same is true. The voting results of the FDA oncology drug consulting committee oppose the indications approved by Duvelisib approved by overwhelming data.
/ 03 /
The standard of good and bad judgment of tumor drugs is inseparable from the "gold standard"
Pi3K inhibitors rollover also tells us that although the clinical replacement end is good, it is not the standard for our 100 % trust.
Various facts have proved that these clinical replacement end and total survival period cannot be completely drawn.
Take the commonly used clinical replacement endpoint ORR, it can reflect the degree of the sum of the diameter of the tumor lesion than the level of the baseline, which is a key indicator in the evaluation standard of the physical tumor. Therefore, the improvement of objective relief rate can indeed show that patients have a positive response to treatment.
However, the relationship between ORR and OS is not so related. Although some tumor drugs can increase ORR in the early stage, if drug -resistant tumors may progress faster, it will not improve OS.
In addition, the effects of side effects on the survival of tumor patients cannot be reflected through ORR. The case of PI3K inhibitors using clinical replacement ending is the best example.
In fact, the example of the use of clinical replacement ending is not limited to PI3K suppression.
Many of the 7 retracted drug indications that were accelerated by FDA in 2021 were because the early ORR data could be considerable, but the overall survival period did not show the advantage.
For example, the third -line treatment of K -drug was previously accelerated for small cell lung cancer, and the basis is an objective relief rate.
However, in March 2021, because in subsequent clinical trials, the efficacy of the first line of the first line of SCLC patients using the K -drug+EP solution and placebo+EP solution showed that the OS of the K drug group did not meet statistically significance. This indicator was withdrawn. Essence
Of course, in the treatment of PD- (L) 1, there are some special circumstances: presenting significant overall survival benefits, but there is no great effect on the ORR indicator. Therefore, ORR cannot accurately reflect the changes of OS.
Anyway, perhaps the clinical replacement end point can help the drug first, but it is difficult to go to the end without OS data.
As statistics scientist Stephen Senn said:
"The relationship between replacement end and real end is like checks and cash. You can get a check in advance, but you may also encounter a refund."
Disclaimer: This article is the content of Yaozhi.com, and the copyright of the pictures and text belongs to the original author. The purpose of reprinting is to pass more information, which does not represent the viewpoint of this platform. If the content, copyright and other issues are involved in the work, please leave a message on this platform, and we will delete it as soon as possible.
- END -
Emergency!Involving a funeral

On the evening of September 24th, Zhuji City, Shaoxing, Zhejiang Province reported...
The symptoms of a case in Beijing have not been reported in time, and many renewal infections have been caused
On June 19, Beijing reproduced the infected infected with society.According to Bei...