The 5 -year survival rate is nearly doubled!Immunotherapy reached a new high | 2022 ESMO
Author:Cancer Channel of the Medical Time:2022.09.19
*For medical professionals for reading reference

PD-L1 single medicine and combined chemotherapy treatment of lung cancer progress
The 2022 ESMO Conference was held in the online and offline online and offline online and offline in Paris, France from September 9th to 13th, 2022. In terms of lung cancer treatment, non-small cell lung cancer (NSCLC) and small cell lung cancer clinical trials were reported. Whether in PD-L1 single drug treatment or PD-L1 combined chemotherapy for treatment, patients' survival indicators have improved.
The "Medical Tumor Channel" specially shared on three studies ~
Clinical Question 1: Can NSCLC use PD-1 inhibitors after surgery, can it exempt chemotherapy?
:Pearls Study: NSCLC patients can use Paborizumab for average of Pabblitzab for auxiliary treatment regardless of PD-L1 expression level
Summary number: 930Mo
Pearls/Keynote-091 test is a three-blind random control-phase clinical trial, which aims to evaluate the effectiveness and effectiveness and effectiveness and effectiveness and effectiveness and effectiveness and effectiveness and effectiveness of Paborizumab as the effectiveness and the effectiveness and effectiveness of patients with phase IB (T ≥ 4 cm) to IIIA. safety.
Patients in the group were randomly distributed to the Paborzab's or placebo group at a ratio of 1: 1. The main endpoint of the Keynote-091 test is the disease-free survival (DFS) of the intentional treatment crowd (ITT) and PD-L1 TPS ≥ 50%.
The results showed that Paborizumab significantly extended the DFS of the completely removed period of IB (T ≥ 4cm) to the IIIA phase NSCLC. Analysis in the second period shows that of the two Asian groups of 1%-49%and TPS <1%of TPS, the median and long-term DFS of the Paborizumab group have improved, but among TPS ≥ 50%of the population, The improvement of DFS is not significant in statistics. This may be because the number of people in the group and the additional role of placebo play an additional role.
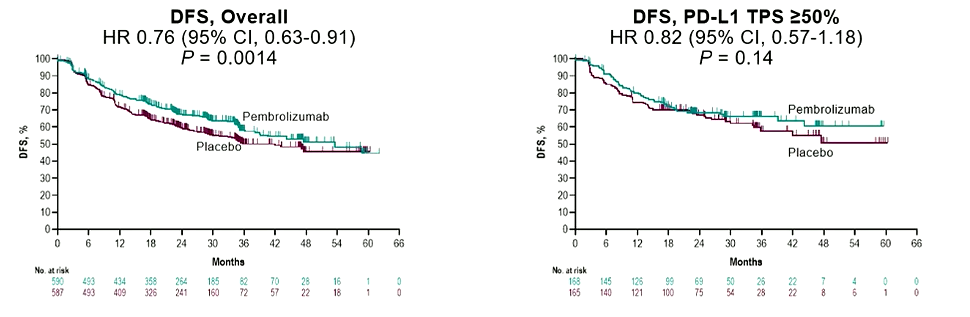
Figure 1 PEARLS test shows that the overall DFS of patients who received Paborizumab were improved, but there was no significant difference in DFS in TPS ≥50%of people
Researchers announced that they will calculate the DFS in the PD-L1 in the next IA. Overall, data supports Paborzab is beneficial to the completely removed IB to IIIA phase IIIA phase IIIA. If the patient needs to be auxiliary chemotherapy, then no matter what the PD-L1 expression is expressed, Paborzumab can be recommended. treat.
In addition to the progress of single drug treatment, PD-L1 combined chemotherapy has also proven to improve the survival of patients with lung cancer.
Clinical Problem 2: What is the beneficiary of survival benefits?
EKeynote-189 Study: Paborzab's combination with Pemeusse and Platinum improved non-scale NSCLC patients survival
Summary number: 973MO
Non -small cell lung cancer can be divided into scales and non -squamous. The pathological type of non -squamous non -small cell lung cancer is mainly adenocarcinoma. At present, some progress has been made in the treatment of non -squamous non -small cell lung cancer patients.
KEYNOTE-189 Study (NCT02578680) is a randomly controlled phase III clinical trial. The patients who are admitted to the group are non-squamous non-small cell lung cancer. The patient accepts 200mg or placebo (Parbryzumab per 2: 1 ratio (2: 1 ratio ( Q3W) Treatment (up to 35 cycles) combined with Pemeusse and platinum (cisplatin or card platinum) treatment for 4 cycles. At the ESMO conference, the researchers reported the results of 5 years of follow -up.
The results showed that Parbilizumab+Pemeuscer+Platinum is compared to Permelus+Platinum, which significantly improves the OS rate of patients with the initial treatment of non -scale NSCLC, and does not cause EGFR /Alk changes.
Among the 616 patients, the median follow -up time of (March 8, 2022) was 64.6 months. 57.4%of patients who received treatment transitioned from the placebo group to anti-PD-(L) 1 treatment during the study. The median OS of the two groups of patients was 22.0 months and 10.6 months, and the 5 -year OS rate was 19.4%and 11.3%, respectively. The mid-position PFS is 9.0 (8.1-10.4) and 4.9 (4.7-5.5) a month (HR, 0.50; 95%CI, 0.42-0.60), and 5 years of PFS are 7.5%and 0.6%.

Figure 2 Parbilizumab+Peime Qucei+platinum treatment patients for 5 years OS is significantly higher than Patients with placebo+Pemeter+platinum chemotherapy (19.4%vs 11.3%)
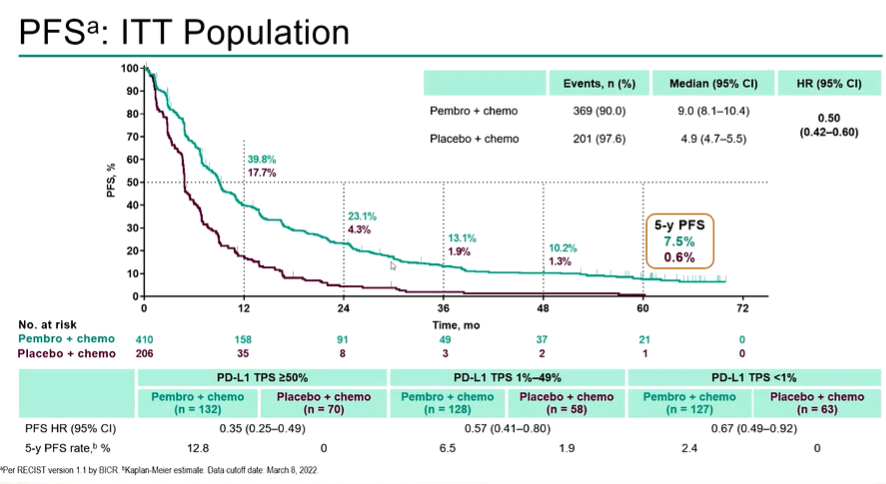
Figure 3 Popalzumab+Peime Qucei+platinum treatment patients for 5 years PFS is significantly higher than the placebo+Permeusus+platinum chemotherapy (7.5%vs 0.6%)
In patients with ≥1 doses of the treatment, 72.8%and 67.3%of patients occurred 3-5 AE. Of the 57 patients who completed the 35 -cycle Paborzab's treatment, the objective relief rate (ORR) was 86.0%(CR, n = 8; PR, N = 41); completed the 35 cycle Pabberzuzhu order The overall survival rate of anti -therapy patients was 71.9%.
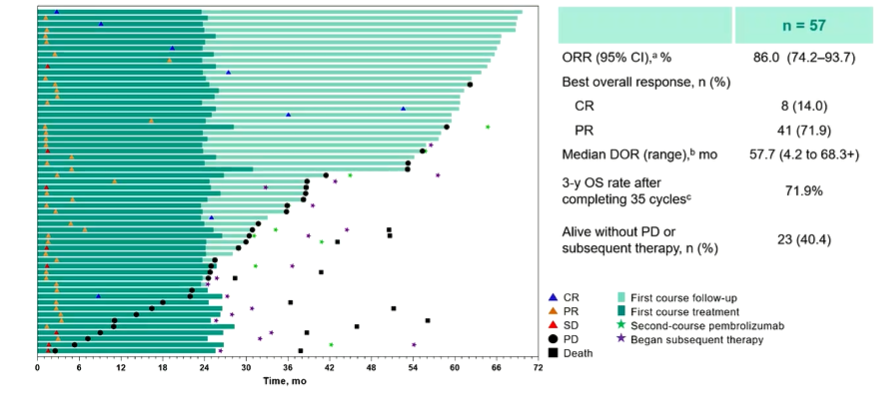
Figure 4 The ORR of patients with 35 cycles of Paborzab+chemotherapy is 86%
Regardless of the expression of PD-L1, Pabaolin Monsteri+Pemeter+Platinum and Platinizer+Pemeter+Platinum have significant improvement in OS and PFS, and the toxicity is controllable. Of the patients who completed 35 cycles of Paborzab's treatment, immunotherapy+chemotherapy has a long -lasting effect. These data further support the treatment standards of Paborzab+Pemeter+platinum as the treatment standard for metastatic non -scale NSCLC, and will not make EGFR/ALK sensitive. Clinical Problem 3: Is the ingredients of the first -line treatment of immunotherapy+chemotherapy NSCLC?
EKeynote-407 Research: Paborzab's combined chemotherapy and individual chemotherapy significantly improved metastatic squamous NSCLC patients from OS and PFS
Summary number: 974Mo
In addition to non -squamous non -small cell lung cancer, immunotherapy combined chemotherapy also won another game in treating squamous small cell lung cancer.
Keynote-407 Research (NCT02775435) is also a phase III clinical trial with a random control. Researchers in this ESMO conference report on the people who intend to treat the crowd (ITT) and complete the 35 cycle (2 years) patients Five -year follow -up results.
The patients who were admitted were non-squamous non-small cell lung cancer. Eligible patients randomly received 200 mg or placebo treatment of Paborzumab at a ratio of 1: 1. Q3W treatment 4 cycles.
Compared with the placebo+chemotherapy, Paborzab+platinum chemotherapy (chemotherapy) significantly extended OS and PFS of patients with unprepared metastatic scale NSCLC. The main ending is OS and PFS.
As of February 23, 2022, the mid-bit time from the beginning to the data deadline was 56.9 (49.9-66.2) a month. The other 26 patients received the follow-up anti-anti-PD-(L) 1 treatment, with an effective cross rate of 51.1%.
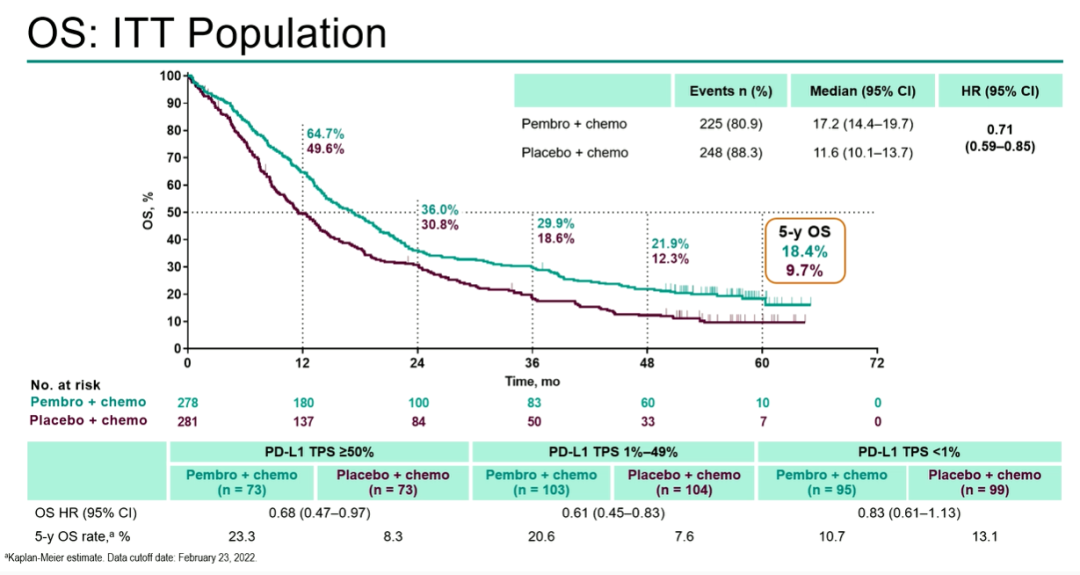
Among the ITT crowd, the median OS of Paborzab+Chemotherapy Group is 17.2 months, and the placebo+chemotherapy group is 11.6 months (HR, 0.71; 95%CI, 0.59-0.85). The 5-year OS of the two groups of patients was 18.4%and 9.7%, and the incidence of 3-5 AEs was 74.8%and 70.0%, respectively.
Figure 5 Poporizumab+chemotherapy patients for 5 years OS are significantly higher than comfortables+chemotherapy patients (18.4%vs 9.7%)
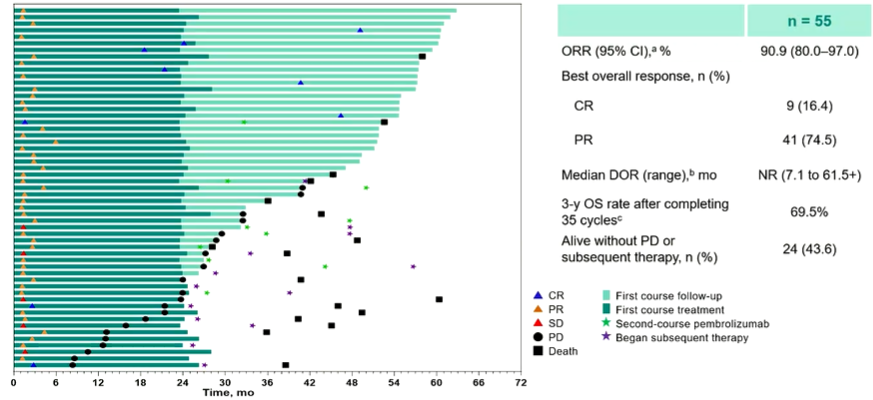
Of the 55 patients who completed 35 cycles of Paborzab's anti -anti -resistance, ORR was 90.9%and OS was 69.5%in 3 years.
Figure 6 The ORR of patients with 35 cycles of Paborzab is 90.9%, and 3 years of OS are 69.5%
It can be seen that after 5 years of follow -up, compared with individual chemotherapy, OS and PFS of Paborizumab combined with chemotherapy are significantly extended, and the toxicity has not increased. Most patients who complete 35 cycles have an objective relief and survived at the end of the data. These results have proved that Pabberzumab+chemotherapy can be used as a standard first -line treatment option for metastatic scale NSCLC.
As of now, the above three experiments have achieved phased results. The test results show that PD- (L) 1 monoclonal antibody can improve the survival of lung cancer patients to a certain extent in terms of single drug treatment and combined chemotherapy. You can see that you can see PD- (L) 1 Monopoly has good application prospects in the treatment of lung cancer.
The first release of this article: the medical world tumor channel
Author of this article: Yan Hao
Editor in charge: Sweet
- END -
[Prevention and control of the epidemic] All members of the Santai County Market Supervision Bureau of Mianyang City returned to work and went to the front line together

In order to further do a good job of preventing and controlling the epidemic, on S...
The event trajectory is announced!Nine new cases of new coronary pneumonia are added to Shiyan City

justShiyan City Health and Health CommitteeRelease the latest noticeDetails are as...