good news!343 kinds of medicines are expected to be included in medical insurance
Author:Hubei Daily Time:2022.09.18

good news!
343 medicines
Formally pass formal review
It is expected to be included in the national medical insurance catalog
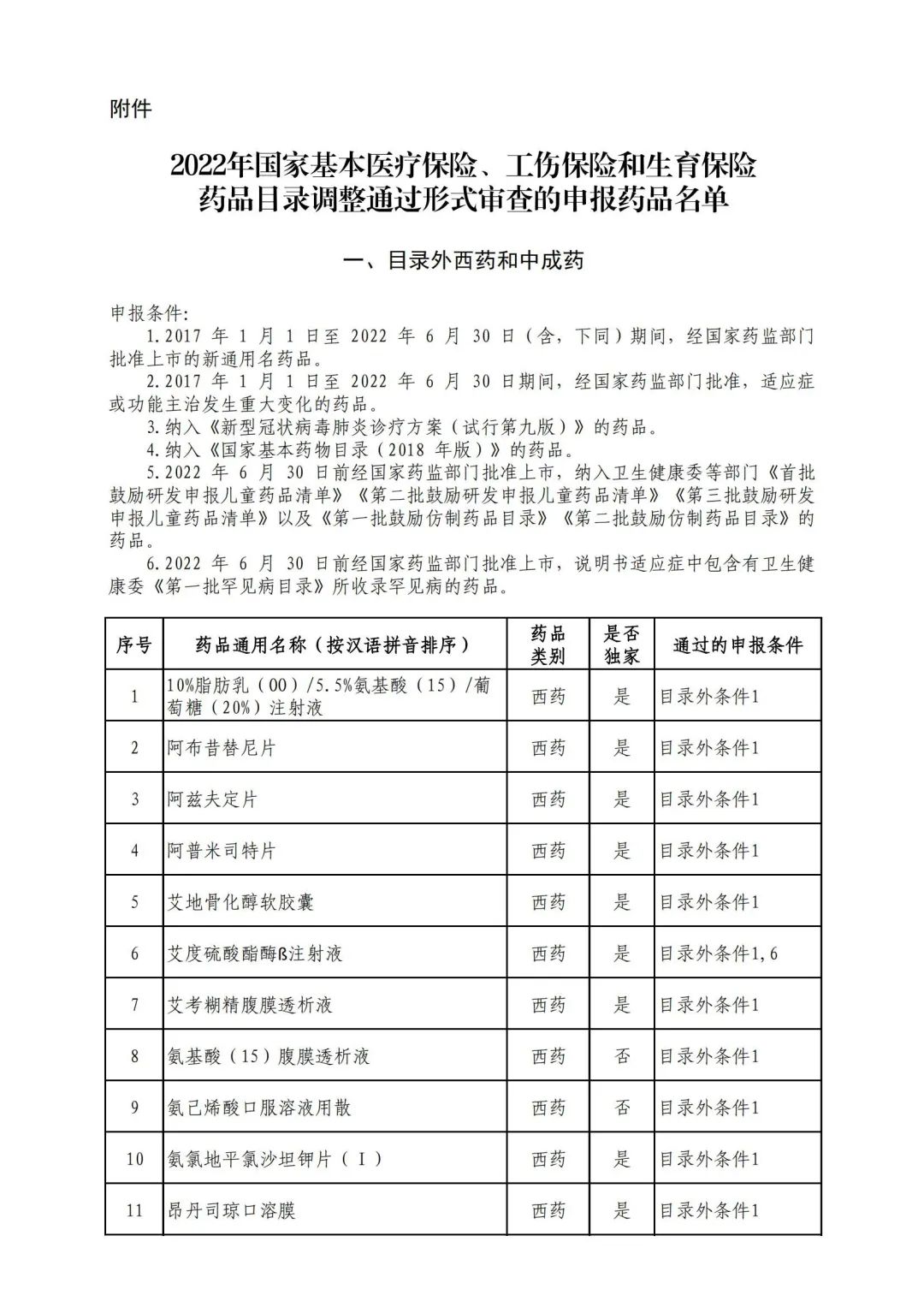
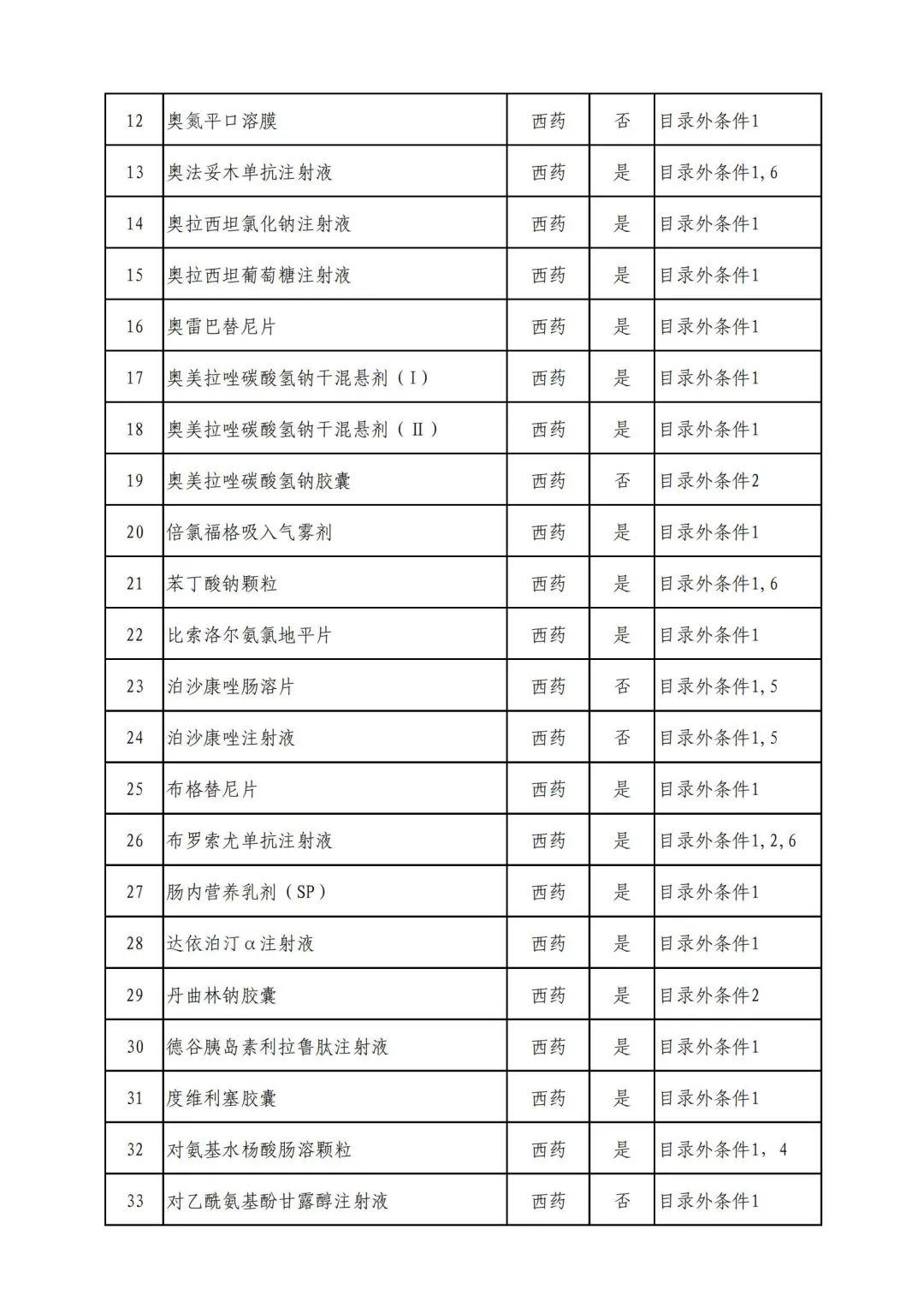
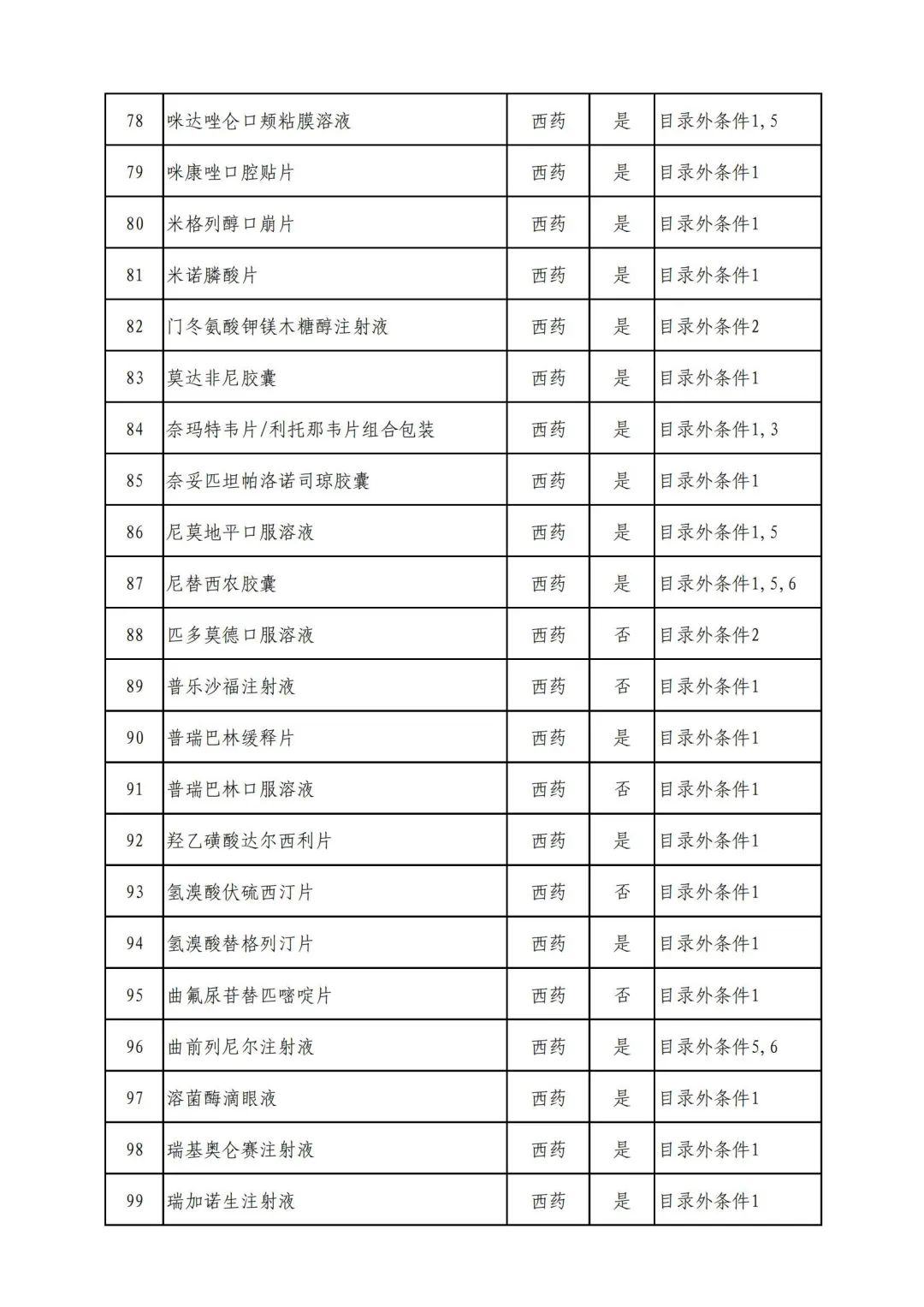
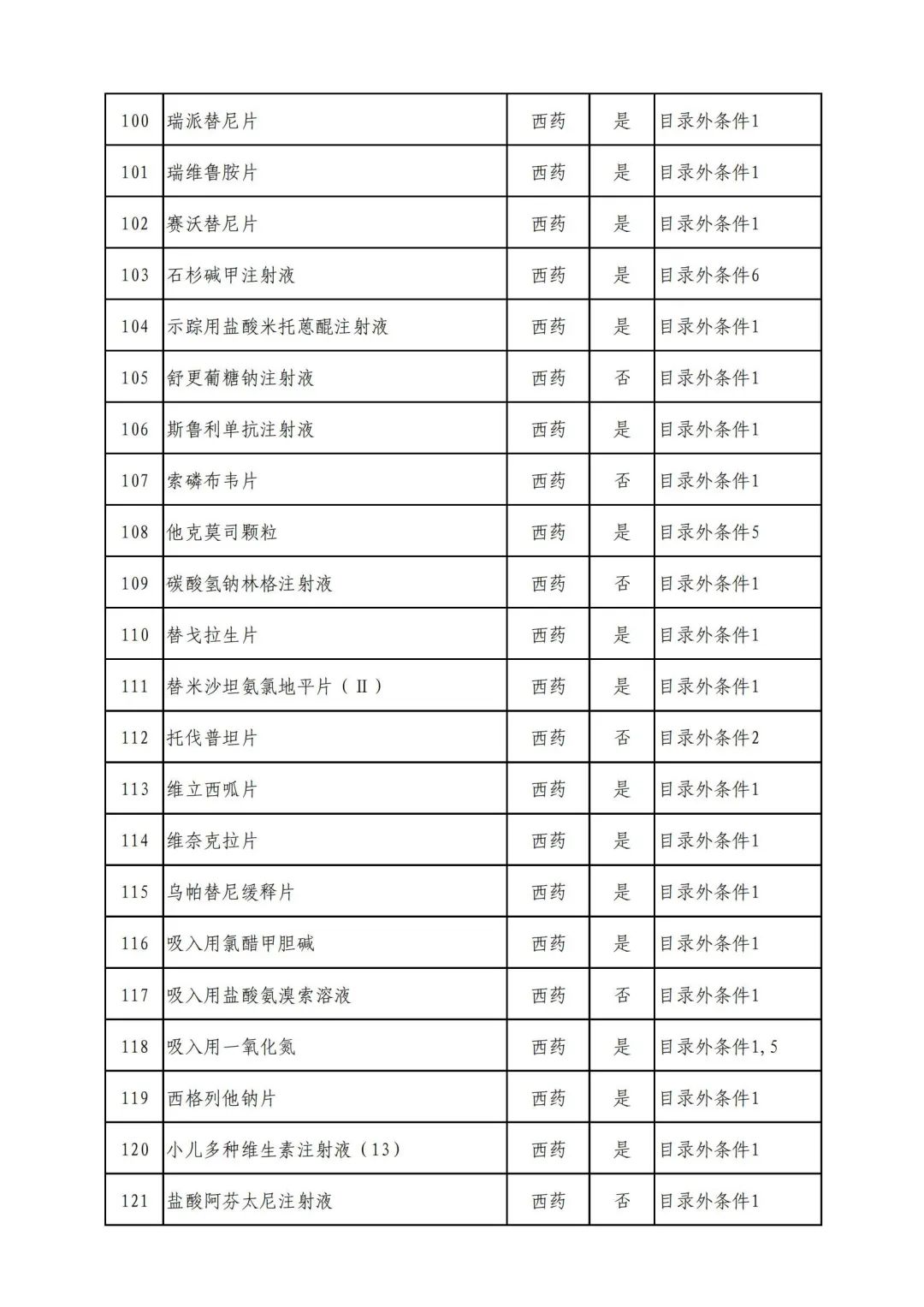
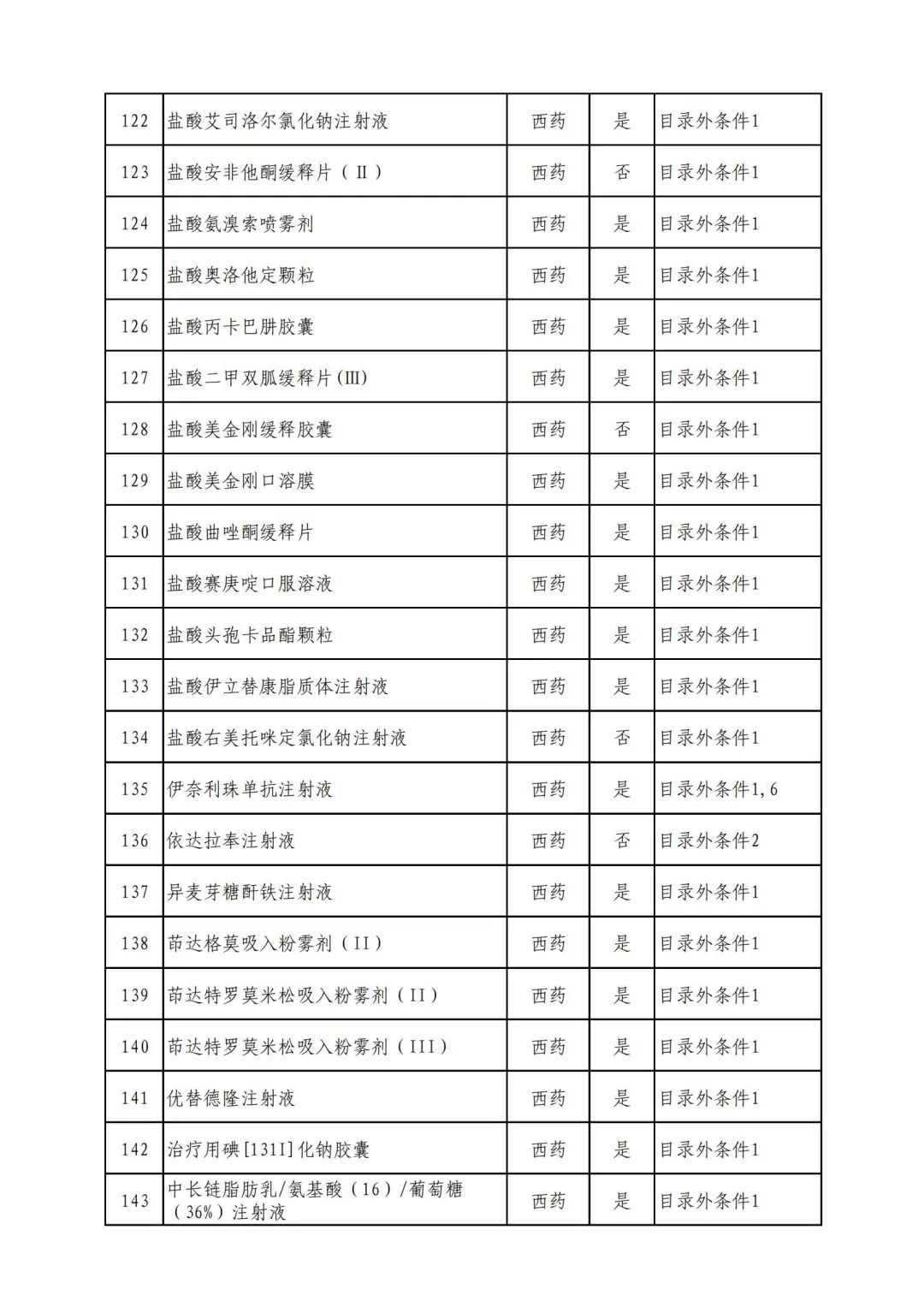
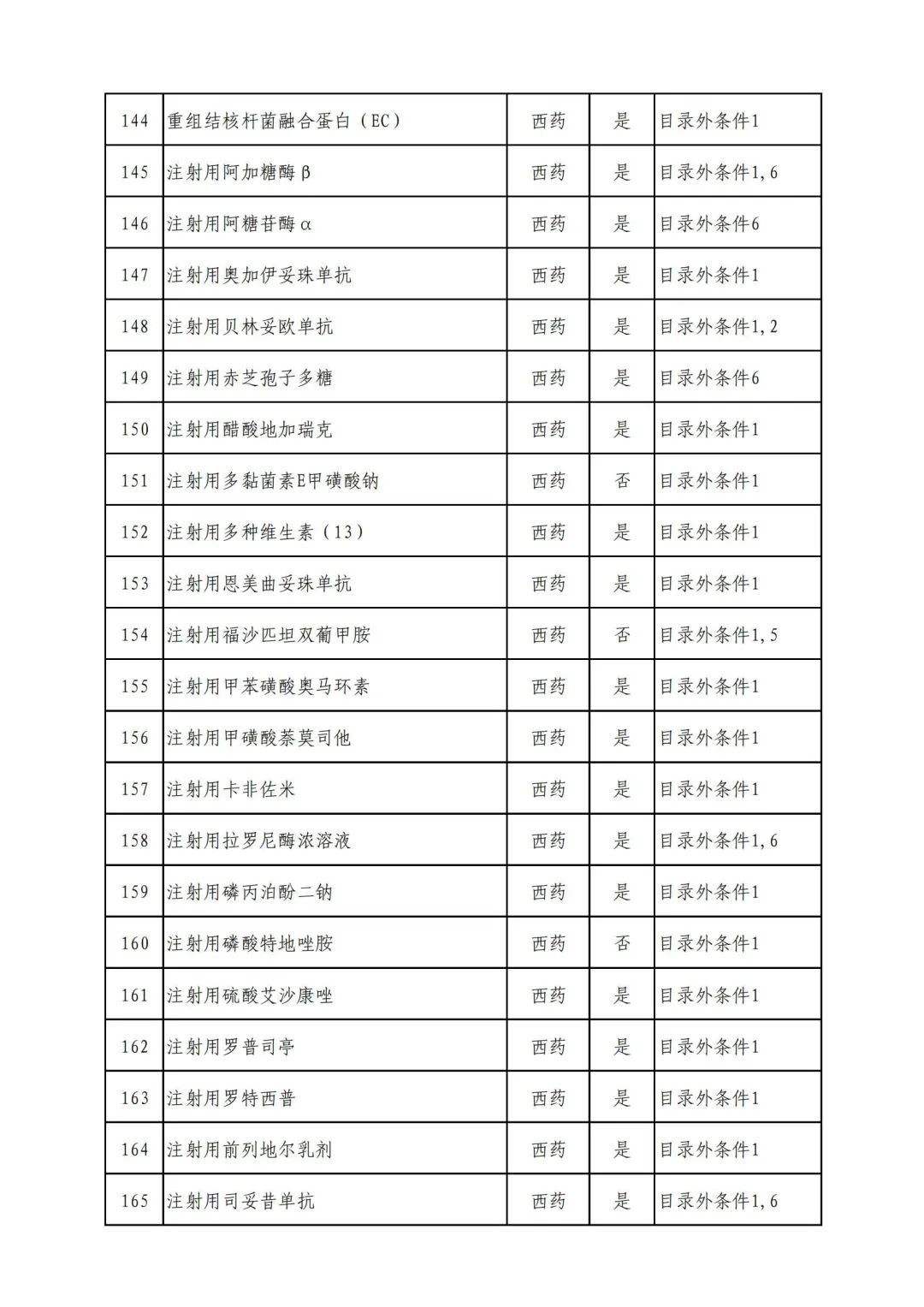
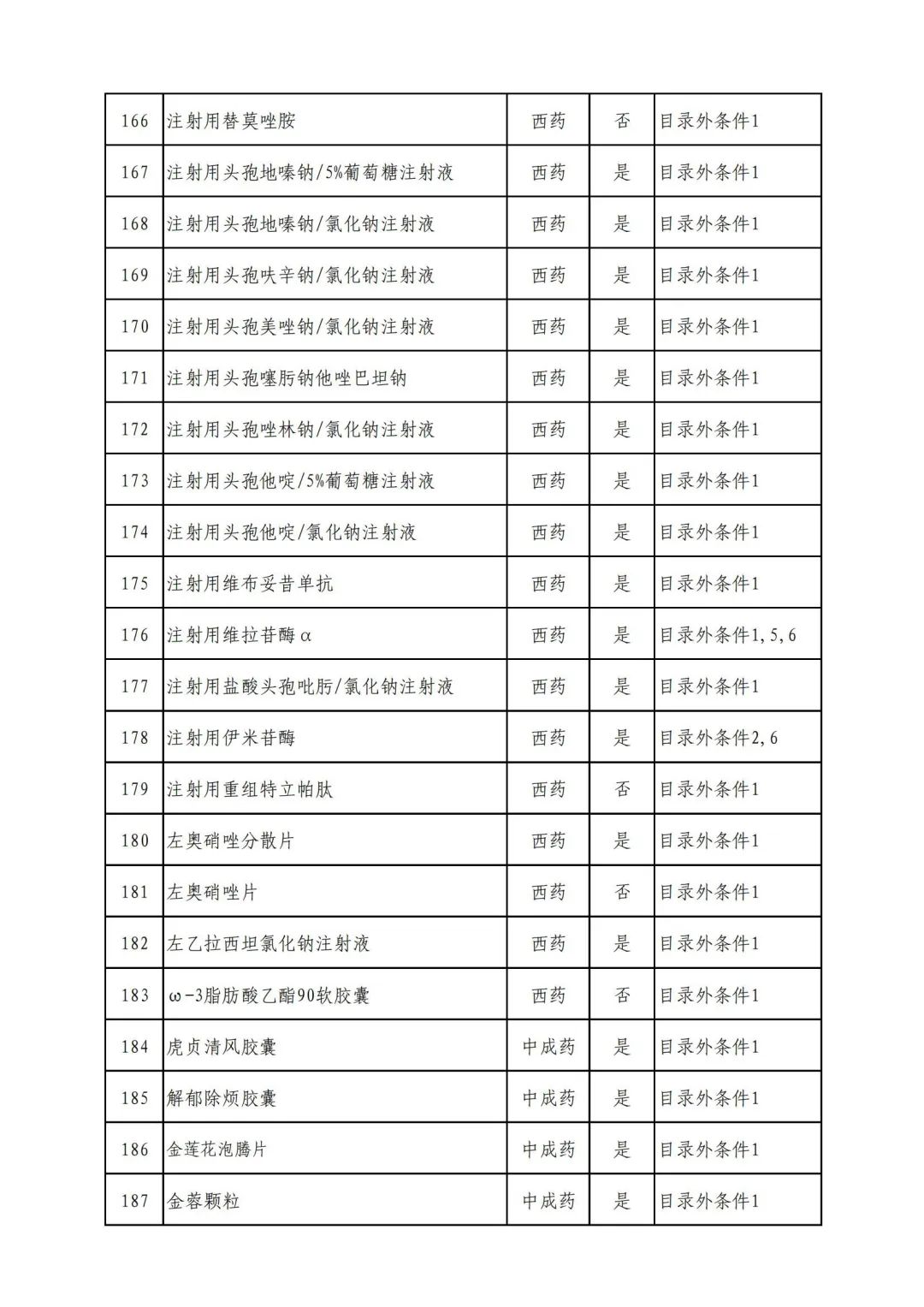
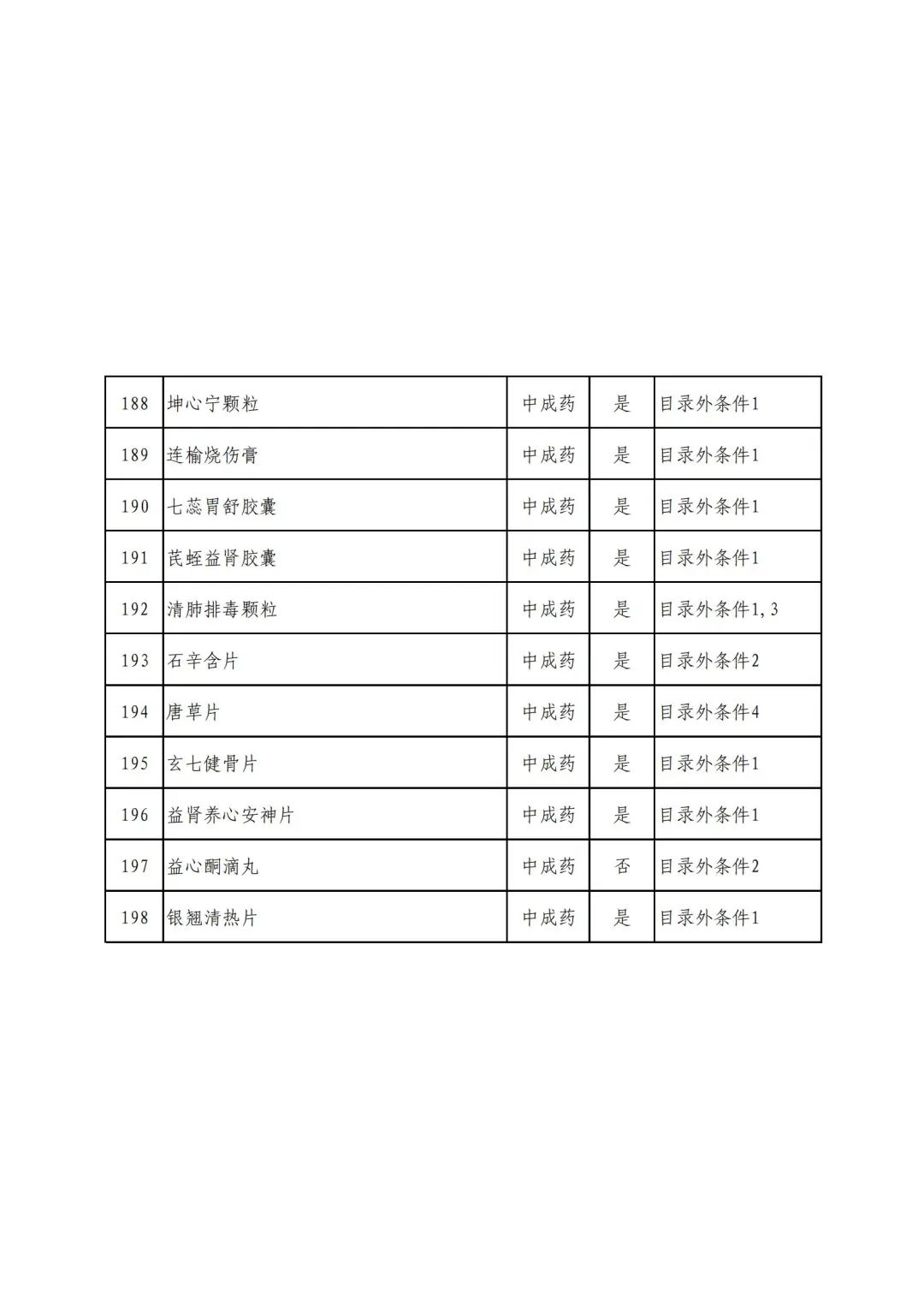
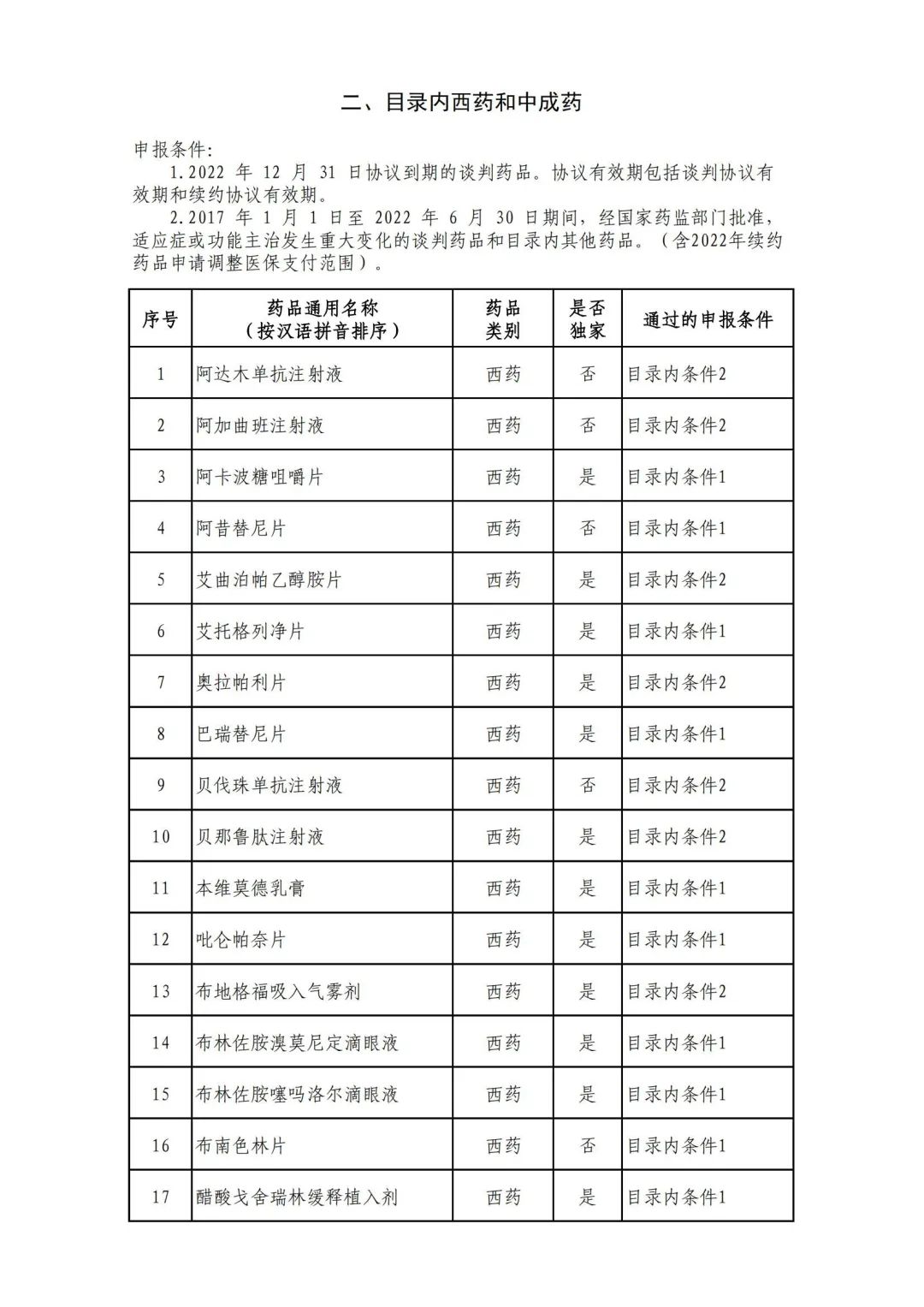
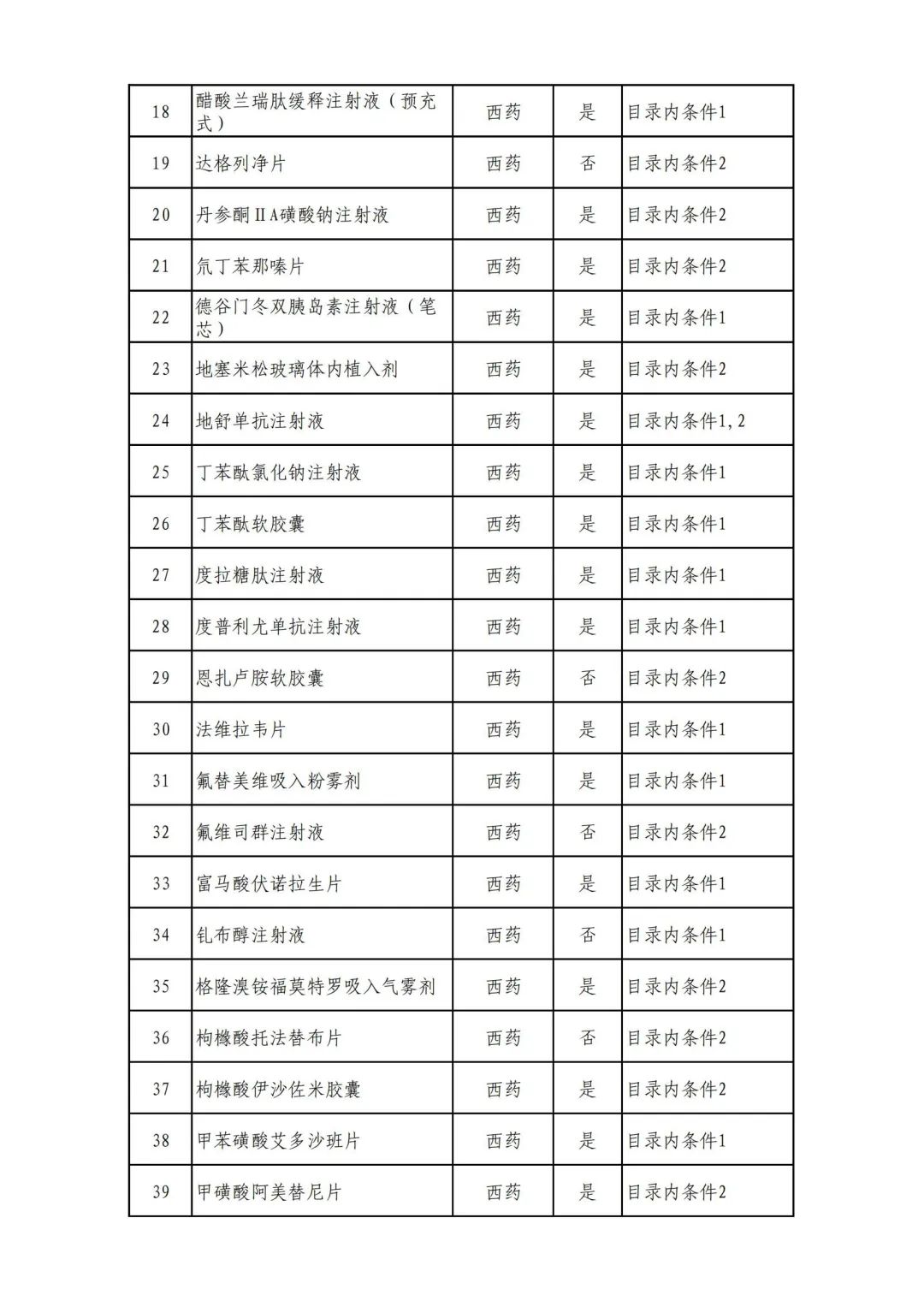
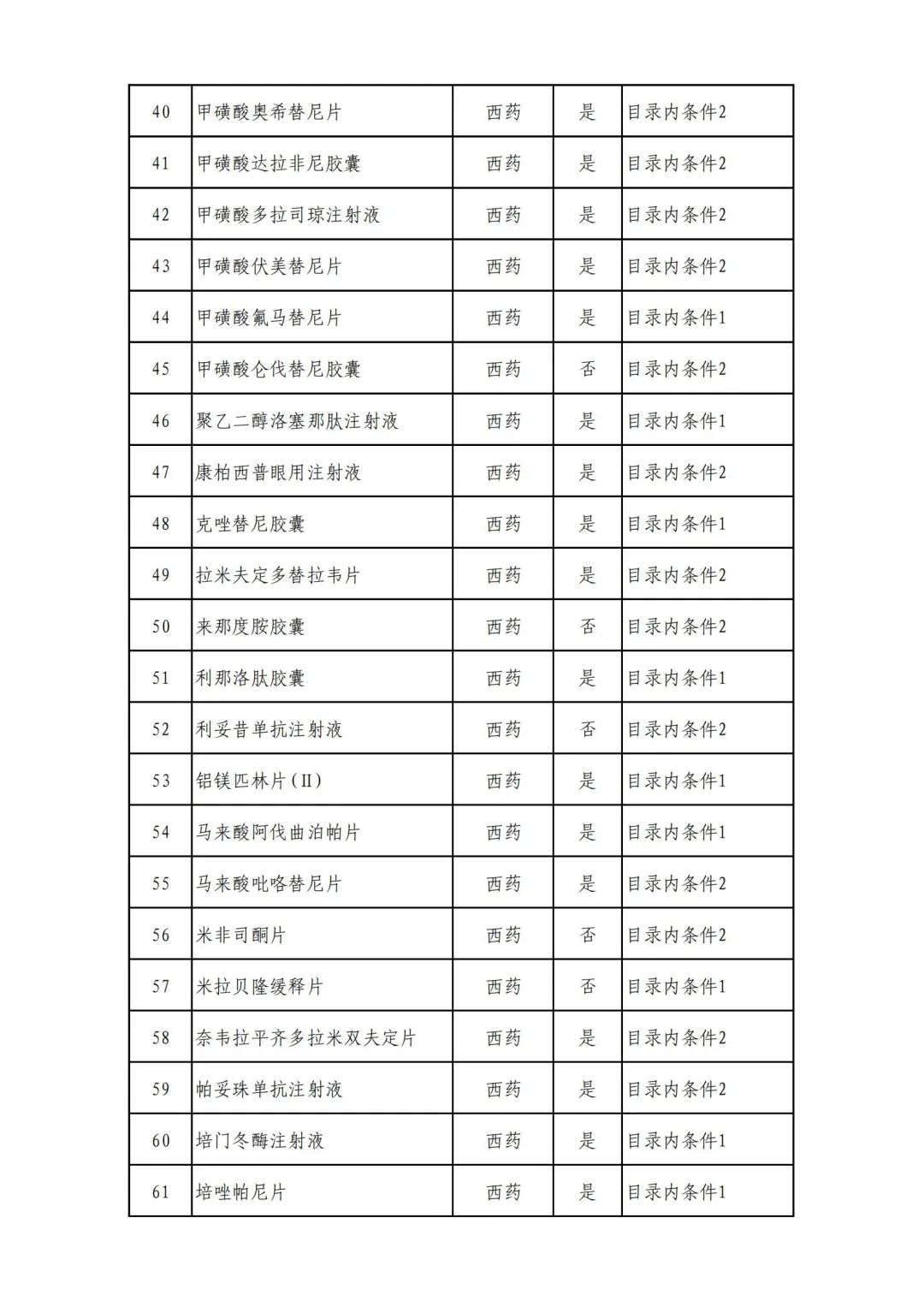
On September 17, the State Medical Insurance Bureau officially announced the "List of Application Drugs for the Adjustment of National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug Catalogs in 2022, and a total of 343 drugs were officially reviewed.
Plus a big picture on the ups and downs to see the list of medicines


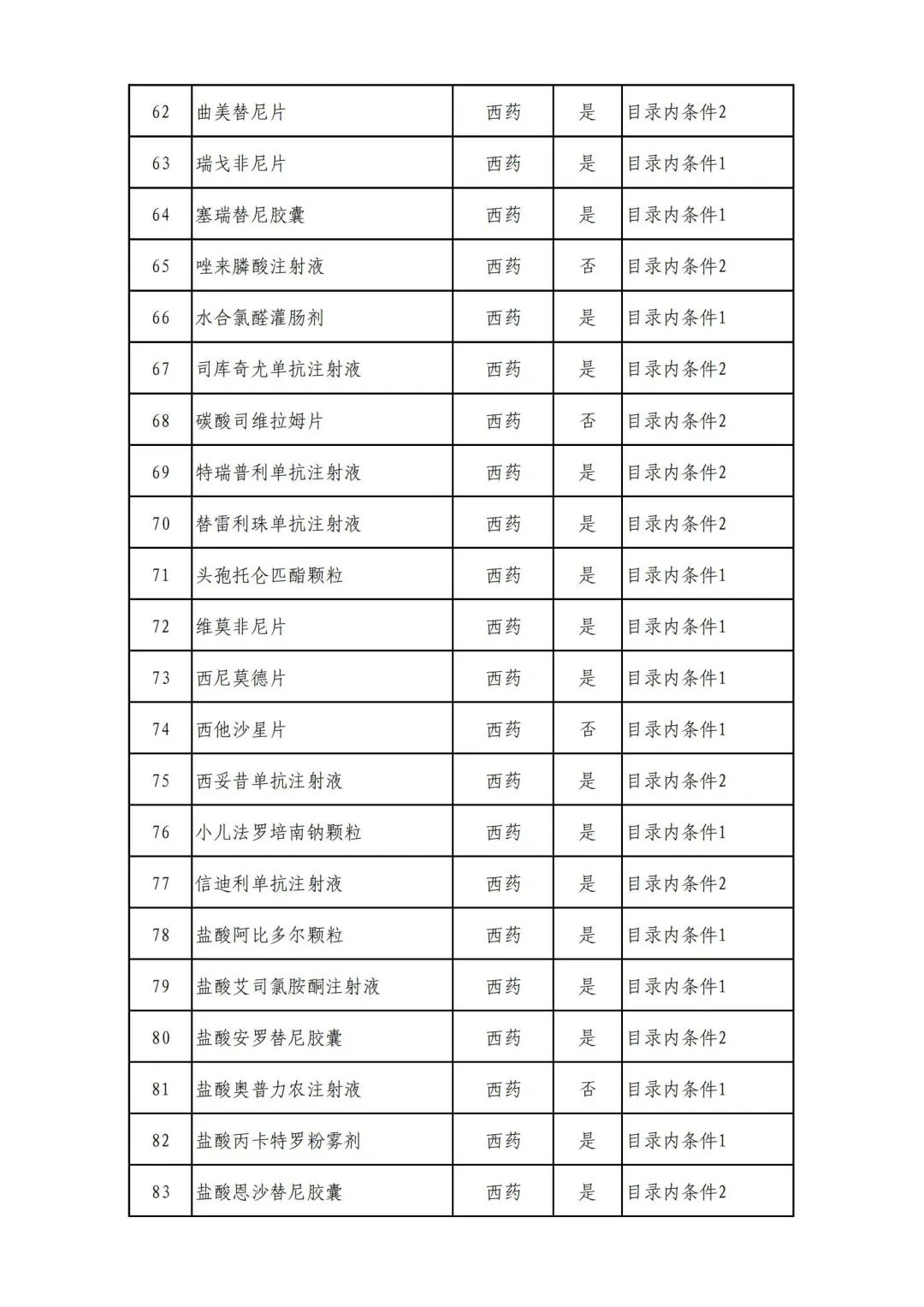
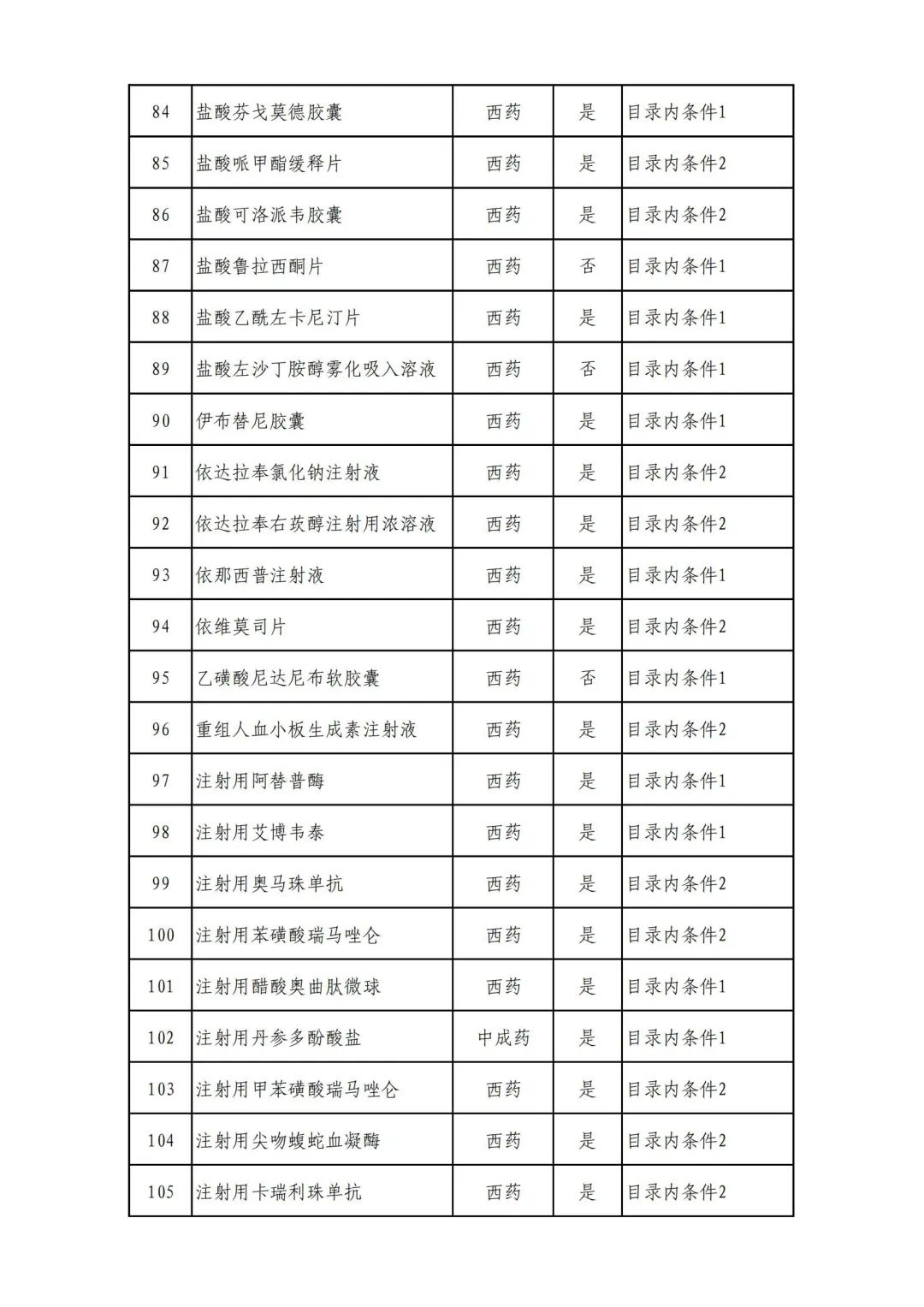

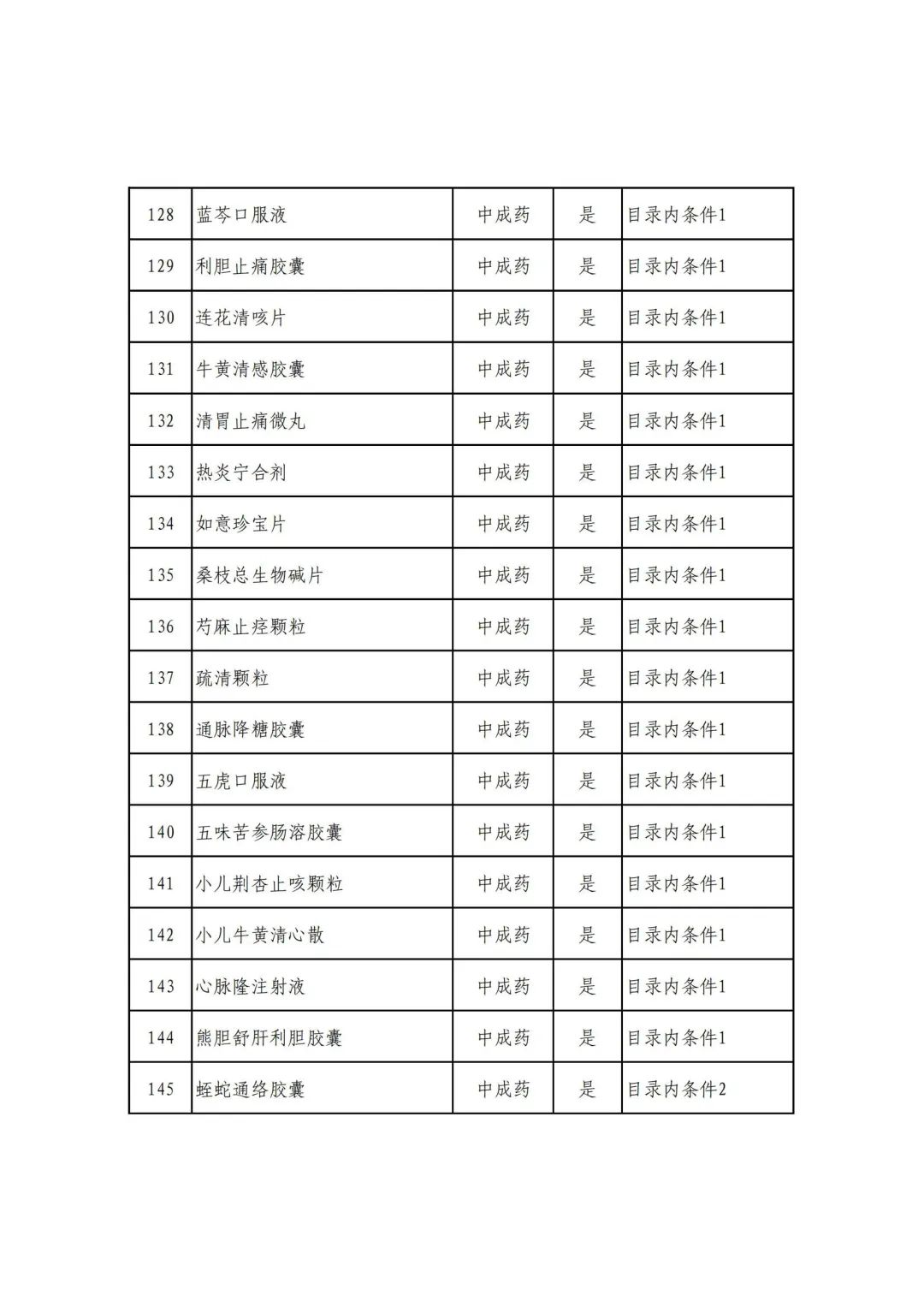
Some new coronary pneumonia therapy drugs
Through form review
In 2022, a total of 343 kinds of drugs were reviewed in the list of application drugs, including the list of national basic medical insurance, work injury insurance and maternity insurance drugs. Western medicines and Chinese medicine and Ada Mipidica injection, including 145 types of catalogs and Chinese medicines, and Chinese medicines. Compared with 344 drugs on the list of drug lists that have been reviewed by preliminary forms, 1 kind of western medicine -metrica vaginal expansion inflation was reduced bolt.
In the context of the normalization of the new crown epidemic prevention and control, from the first implementation of the independent application system of the medical insurance drug directory enterprise in 2020, the State Medical Insurance Administration attaches great importance to the treatment of new crown pneumonia. "Pharmaceuticals" as one of the application conditions, a number of new crown pneumonia treatment medication has been included in the medical insurance drug catalog. The National Medical Insurance Administration stated that during the application process, the new crown pneumonia therapy drugs that have been declared and reviewed through formal review will carry out follow -up work in accordance with procedures and strive to officially include the medical insurance directory at a reasonable price.
A variety of rare diseases
Through form review
In July, the National Medical Insurance Bureau stated that there was no time limit for the launch of the medical insurance directory for the "approved listing after January 1, 2017" for rare diseased drugs. As early as this year, when the medical insurance directory negotiation work was launched, the State Medical Insurance Bureau made it clear that the medical insurance directory would appropriately tilt to special groups such as patients with rare diseases and children. The list of drugs that passed the formal review contains a number of rare diseases, such as spinal muscle atrophy (SMA) therapeutic pharmaceutical Liste Polan oral solution, type Ⅰ Gobei disease treatment drug injection for hemididin, hemidin, Ease enzymes, type Ⅰ adhesive polysaccharides, special effects of adhesive and accumulation of Roninase, adurtlase enzyme enzyme α, etc.
The next step is organized according to the procedure
Expert review and other work
The National Medical Insurance Bureau stated that the adjustment of medical insurance and drug catalogs is divided into aspects of corporate application, form review, expert review, negotiating bidding, etc. The formal review is only one of them. Through form review, the drug is eligible to enter the next expert review session. Only by successfully adjusting the directory adjustment can it be eventually included in the national medical insurance directory.
In the next step, the National Medical Insurance Administration will organize the work of expert review in accordance with the requirements of the "Interim Measures for the Management of Basic Medical Insurance Drugs" and "2022 National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug Catalog". If the progress is smooth, the new version of the medical insurance drug catalog will be announced in November, and the implementation of the landing on January 1 next year will be announced.
Source: official website of the National Medical Insurance Bureau
- END -
The transformation of the research and innovation achievements of Chinese medicine linked diseases helps the development of Chinese medicine towards a new era

On July 6, the Management Committee of Shijiazhuang High -tech Industrial Developm...
The latest situation of the epidemic prevention and control of Xinjiang, Tibet, Hainan and other places!(As of 24:00 on August 27)

The National Health and Health Commission released on the morning of August 28At 0...