Latest notice: Safe sales!recall!
Author:Hebei Radio and Television Sta Time:2022.06.21

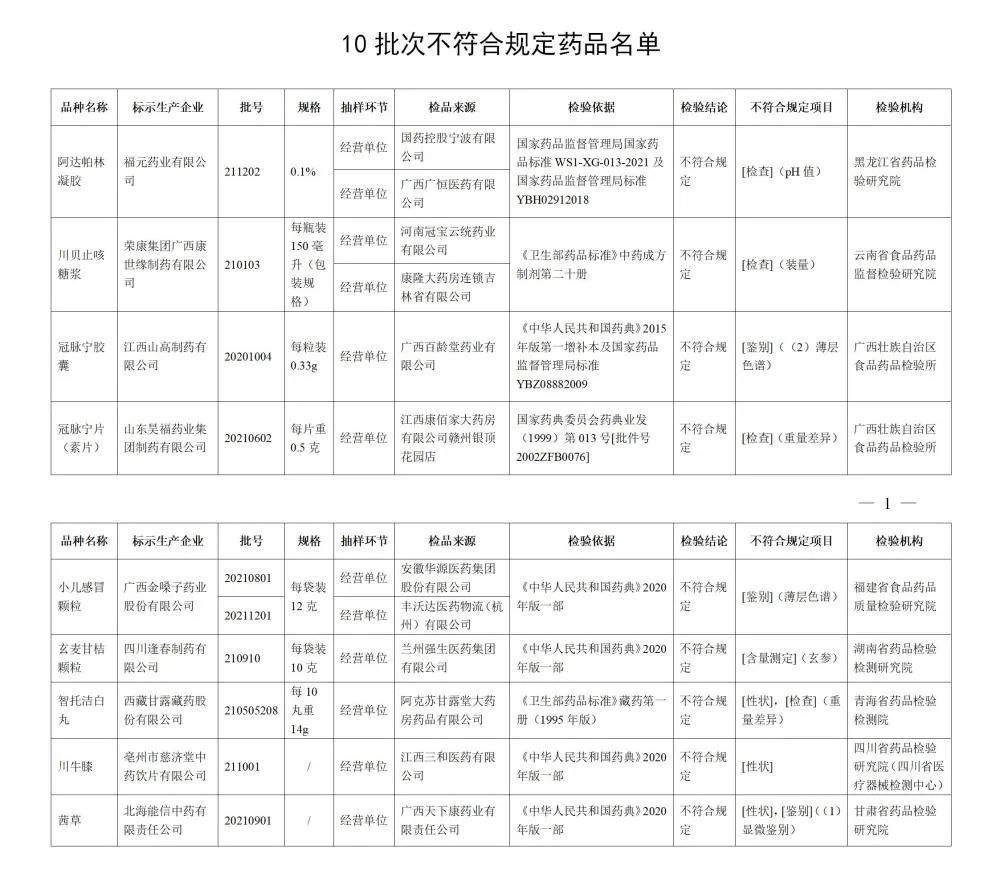
After inspection by eight pharmaceutical inspection institutions including Food and Drug Quality Inspection and Research Institute in Fujian Province, 10 batches of children's cold particles such as Guangxi Golden Voice Pharmaceutical Co., Ltd., including Guangxi Golden Voice Pharmaceutical Co., Ltd., did not meet the regulations. The relevant situation is notified as follows:
I. After inspection by Heilongjiang Pharmaceutical Inspection and Research Institute, a batch of Adapolin gel produced by Fuyuan Pharmaceutical Co., Ltd. does not meet the requirements and does not meet the requirements of the specified project as a pH value.
After inspection by the Institute of Food and Drug Supervision and Inspection of Yunnan Province, a batch of Chuanbei cough syrup produced by Rongkang Group Guangxi Kangshiyuan Pharmaceutical Co., Ltd. did not meet the requirements and did not meet the requirements of the regulations as the installation.
After inspection by the Food and Drug Inspection Institute of Guangxi Zhuang Autonomous Region, a batch of coronal pulse capsules produced by Jiangxi Shan Gao Pharmaceutical Co., Ltd. did not meet the requirements and did not meet the requirements of the regulations as a identification; One batch of coronary Ning Ning tablets (vegetarian tablets) does not meet the regulations, and it does not meet the requirements of the specified project as a weight difference.
It was inspected by the Food and Drug Quality Inspection and Research Institute in Fujian Province. The two batches of children's cold particles produced by Guangxi Golden Vol.
After inspection by the Hunan Pharmaceutical Inspection and Inspection and Institute, a batch of Xuanmai Ganjia particles produced by Sichuan Fengchun Pharmaceutical Co., Ltd. did not meet the requirements, and did not meet the requirements of the specified project as the content measurement.
After inspection by the Qinghai Pharmaceutical Inspection and Inspection Institute, a batch of Zhito Jiejie White Pills produced by Tibetan Ganlu Tibetan Pharmaceutical Co., Ltd. did not meet the regulations and did not meet the requirements of the specified projects as traits and weight.
After inspection by the Sichuan Pharmaceutical Inspection and Research Institute (Sichuan Medical Device Inspection Center), a batch of Sichuan Achyranthes knee produced by Tzuchi Tzuchitang Traditional Chinese Medicine Crimination Co., Ltd. did not meet the regulations and did not meet the requirements of the specified projects as traits.
After inspection by the Gansu Provincial Academy of Pharmaceutical Inspection and Research, a batch of Qiancao produced by Beihai Nengxin Traditional Chinese Medicine Co., Ltd. does not meet the requirements and does not meet the requirements of the specified projects as traits and identification.
2. For the above -mentioned do not meet the prescribed drugs, the drug supervision and management department has required relevant enterprises and units to take risk control measures such as suspension of sales, recall, and recall, and conduct investigations and rectification on reasons for not compliance.
3. The State Drug Administration requires relevant provincial drug supervision and management departments to organize investigations on suspected illegal acts existing above -mentioned enterprises and units in accordance with the "Drug Administration Law of the People's Republic of China", and publicize the results of the investigation and punishment in accordance with regulations.
announce.
appendix:
1.10 batches do not meet the specified drug list
2. Do not meet the specified projects of the project
State Drug Administration
June 9, 2022
- END -
The darker the egg yolk, the more nutritious?Doctor: Most people have eaten the wrong, the truth is ...
Special Author: Liu Pingping | Registered Nutritionist Painter: My Neighbor Totoro...
"No" to obesity!How to lose weight for patients with medium and severity obese?

In recent years, with the increasing number of obese people, the three hospitals i...