Self -check!These 17 batches of medicines do not meet the requirements
Author:Gansu daily Time:2022.09.16
After inspection by 4 pharmaceutical inspection institutions such as Hubei Pharmaceutical Supervision and Inspection and Research Institute, 17 batches of drugs such as acetylcysteine injections produced by Shanxi Guorun Pharmaceutical Co., Ltd. have not met the regulations. The relevant situation is notified as follows:
I. After inspection by the Hubei Provincial Institute of Drug Administration, the two batches of acetylcysteine injection produced by Shanxi Gurun Pharmaceutical Co., Ltd. did not meet the regulations and did not meet the specified projects as hydrogen sulfide.
After inspection by the Jiangxi Provincial Academy of Pharmaceutical Inspection and Inspection, the two batches of Huoxiang Zhengqi water produced by Hubei Tongdutang Pharmaceutical Co., Ltd. did not meet the requirements, and did not meet the requirements of the regulations.
After inspection by the China Food and Drug Inspection Research Institute, it is marked as Hebei Anguo Zhenyu Pharmaceutical Co., Ltd., Shanghai Wanshi Cheng Pharmaceutical Co., Ltd., Shaoxing Zhenyuan Traditional Chinese Medicine Drinking Co., Ltd., Anhui Tongtai Zutang Pharmaceutical Co., Ltd. Shenghai Traditional Chinese Medicine Drinking Co., Ltd., Jiangxi Zhongkang Traditional Chinese Medicine Drinking Co., Ltd., Guangdong Shizhe Pharmaceutical Co., Ltd. and Gansu Guogao Pharmaceutical Co., Ltd. did not meet the regulations, and the project was not in line with the regulations to disable pesticide residues.
After inspection by the Gansu Provincial Academy of Pharmaceutical Inspection and Research, a batch of Qiancao produced by Sichuan Yuancao Traditional Chinese Medicine Drinking Co., Ltd. did not meet the requirements, and did not meet the specified projects including traits and identification.
After inspection by the China Food and Drug Inspection Research Institute, it is marked that 4 batches of authentic purple grass produced by Hebei Honghan Pharmaceutical Co., Ltd., Xinjiang Enze Traditional Chinese Medicine Drinking Co., Ltd. and Huayi Huayi Traditional Chinese Medicine Drinking Factory Co., Ltd. It meets the specified projects as traits.
2. For the above -mentioned do not meet the prescribed drugs, the drug supervision and management department has required relevant enterprises and units to take risk control measures such as suspension of sales, recall, and recall, and conduct investigations and rectification on reasons for not compliance.
3. The State Drug Administration requires relevant provincial drug supervision and management departments to organize investigations on suspected illegal acts existing above -mentioned enterprises and units in accordance with the "Drug Administration Law of the People's Republic of China", and publicize the results of the investigation and punishment in accordance with regulations.
Source: CCTV News

Editor in charge: Yu Shuai
Supervisor: Mu so strong
- END -
Women have a decline in fertility after the age of 30!"Sofele" ovaries may be useful
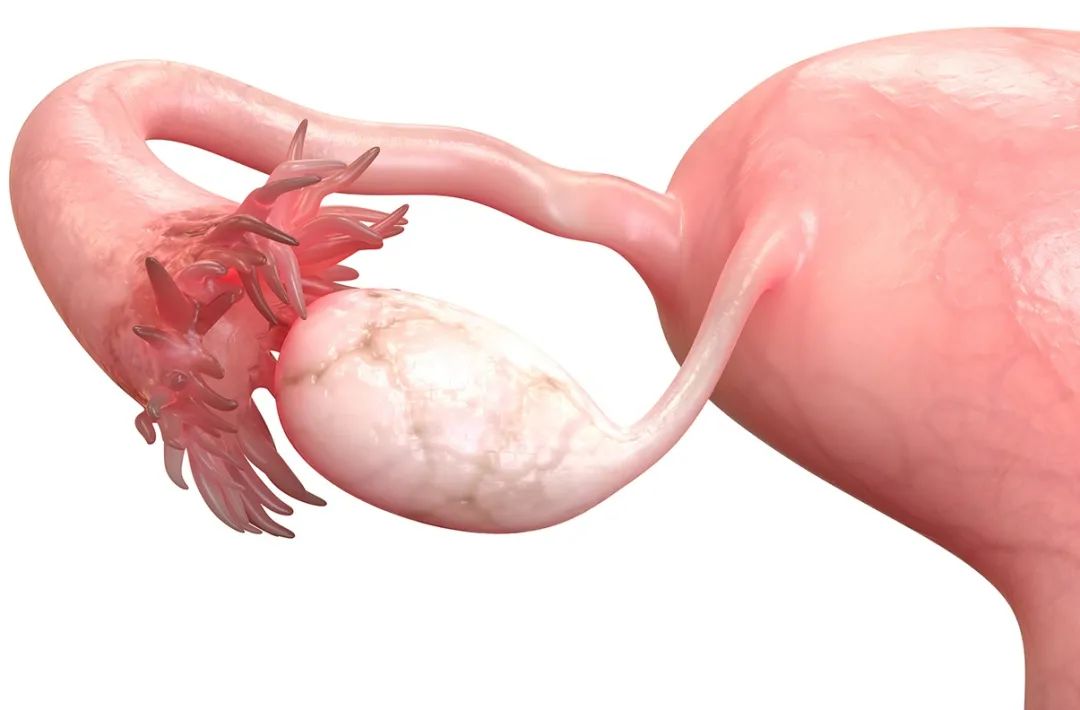
Text | Wang FangImage source: MagicMine/IsstockI hope that the expectant parents wh...
Notice on the test of nucleic acid testing in the fifth round of Pingyao County, Jinzhongzhong County, Shanxi: All are negative

On August 26, our county carried out the fifth round of regional nucleic acid test...