A -share pharmaceutical company R & D strength rankings: Industry research and development investment has increased, Baiji Shenzhou has the most investment, and cutting -edge biological proportion is the largest
Author:Huaxia Times Time:2022.09.15

China Times (chinatimes.net.cn) reporter Wang Yu Yu Na Beijing Beijing reported
R & D strength is the "hard core" indicator of pharmaceutical companies. A few days ago, the reporter of the Huaxia Times carried out statistics on the R & D strength of 232 pharmaceutical companies in A shares, and made research and judgment from special indicators such as the total research and development expenditure and the total research and development expenditure.
my country's pharmaceutical market consists of three major sections: chemical, biopharmaceutical and traditional Chinese medicine, and biopharmaceuticals have the most potential development potential. Junshi Biological Related person told the reporter of the Huaxia Times that the biomedical industry is a capital and technology -intensive industry. Large R & D investment is the key to maintaining core competitiveness of biological innovative drug companies.
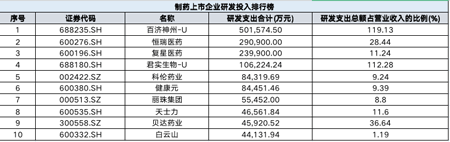
According to Wind data, in the first half of 2022, the total R & D expenditure among 232 pharmaceutical companies was 31 billion yuan, an increase of 12%year -on -year. Among them, the top three companies in R & D expenditure are Baiji Shenzhou, Hengrui Pharmaceutical, and Fosun Pharmaceutical; the total R & D expenditure accounts for the top three companies in the first three of the operating income. Junshi creatures.
The top three in R & D expenditure: Baiji Shenzhou, Hengrui Pharmaceutical, Fosun Pharmaceutical
There is a "thirty law" in the field of biomedicine: the development of a new drug requires at least $ 1 billion in investment, a 10 -year R & D cycle, and a success rate of less than 10%. In the first half of 2022, the total R & D expenditure of 232 pharmaceutical companies in my country was 31 billion yuan, an increase of 12%year -on -year. Among them, the top three companies in R & D expenditure are Baiji Shenzhou, Hengrui Pharmaceutical and Fosun Pharmaceutical.
In the first half of this year, the R & D cost of Baiji Shenzhou was 5.016 billion yuan, an increase of 20.82%year -on -year; the proportion of R & D investment accounted for 119.13%of operating income, an increase of 32.25%year -on -year.
Baiji Shenzhou is a global and in the commercial phase of biotechnology companies. It focuses on research, development, production and commercialization of innovative drugs. At present, there are three drugs that have been independently developed and approved for listing, including a variety of types of drugs, including a variety of types of treatment, including a variety of types of drugs, including a variety of types of drugs, including a variety of types of drugs, including a variety of types of drugs, including a variety of types of drugs, including a variety of types of drugs, including a variety of types of drugs, including a variety of types of drugs, including a variety of types of drugs, including a variety of types of drugs, including a variety of types of drugs, including a variety of types of drugs, including various types Small molecules inhibitors of hematoma, Zibutinib, to treat various physical tumors and anti-PD-1 antibodies for Anti-PD-1 antibodies for Rayleyzumab and small molecule inhibitors Pamipali. At present, Baiji Shenzhou also holds more than 50 pre -clinical research projects. The company said that about half of them have the potential to become the best projects of similar or similar types.
However, Baiji Shenzhou has continued to lose money since its listing. The semi -annual report shows that the company has not made up for a total of 43.994 billion yuan.
Hengrui Pharmaceutical is one of the research and production bases of well -known domestic anti -tumor drugs, surgical drugs, contrast agents and special infusion products. At present, 11 innovative drugs in Hengrui Pharmaceutical have been approved in my country, and more than 60 innovative drugs are developing clinically. More than 260 clinical trials are carried out at home and abroad. Many innovative drug products have achieved global simultaneous development.
In the first half of 2022, Hengrui Pharmaceutical's cumulative R & D investment reached 2.909 billion yuan, an increase of 12.74%year -on -year. Promote to 21.36%. The company said that although the increase in investment in R & D to a large extent has affected the current profit, it provided strong support for the company's long -term development.
In the development of innovative medicines, Hengrui Pharmaceutical has basically formed a virtuous circle of listed batch, clinical batch, and development of a batch of development, and builds a strong independent research and development capabilities.
Fosun Pharmaceutical's R & D investment in the first half of this year was 2.399 billion yuan, an increase of 22.77%year -on -year. Among them, R & D expenses were 1.818 billion yuan, a year -on -year increase of 16.39%.
In the first half of this year, Fosun Pharmaceuticals had 2 innovative drugs (indications), 10 imitation pharmaceuticals (indications) were approved in China/the United States; 1 innovative drug (indicator), 18 imitation pharmaceuticals (indications (indications (indications) (indications (indications) ) Applying for listing in China; 14 innovative drugs (indications) and 9 imitation pharmaceuticals (indications) were approved for clinical trials in China.
Among them, the much -watched Fu Bitai (MRNA new crown vaccine) achieved sales of more than 800 million doses in Hong Kong, Macao and Taiwan in the first half of this year; the first product of Fosun Kate, the joint venture, The first CAR-T cell therapy product, which was approved to be listed, as of the end of July 2022, Yi Kaida has been included in the city's city Huimin insurance and more than 50 commercial insurance in 44 provinces and cities. In July 2022, the holding subsidiary Fosun Pharmaceutical Industry and the real creature established the "Azfdin" joint development agreement, which was exclusively commercialized by Fosun Pharmaceutical Industry Azfdin. Azf's fixed film is the first oral small -molecular crown pneumonia drug that has been approved by my country.
Drawing/Wang Yu
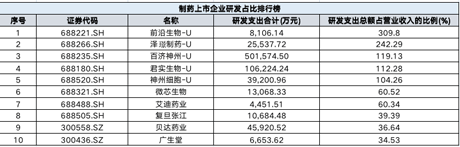
The total amount of R & D expenditure accounts for the top three revenue proportion: cutting -edge creatures, Zezheng Pharmaceutical, Baiji Shenzhou
In Wind statistics, the proportion of R & D expenditures in the first operating income is Yahong Pharmaceutical, First Pharmaceutical Holdings, Maiwei Biology, and League of Pharmaceuticals. Therefore, this indicator is not reference. After excluding the above enterprises, the total R & D expenditure accounts for the top three companies in the first three of the operating income is cutting -edge creatures, Zezhe Pharmaceutical and Baiji Shenzhou, and the fourth place is Junshi creature.
In the first half of this year, the total investment in cutting -edge biological R & D is 081 million yuan, an increase of 44.68%year -on -year; R & D investment accounted for 309.80%of operating income, an increase of 64.19%year -on -year. The company said that the total investment in R & D is increased due to the increase in the R & D investment of FB2001-anti-new coronal virus drugs and FB6001-treatment of drugs for patients with hyperlipidemia.
Achichei, a new type of new medicine developed by cutting -edge biology, is the world's first long -acting anti -HIV fusion inhibitor. In the first half of this year, the company realized operating income of 2,616,100 yuan, an increase of 14.71%year -on -year, and the net profit of the mother -in -law lost 123 million yuan.
Regarding the HIV market, the company said that with the changes in the structure of AIDS patients in my country, patients have become more intense for the effectiveness and safety demands of drugs, and their willingness and ability to buy innovative drugs at their own expense are improved. In addition, more anti -HIV virus drugs are included. Patients with HIV can reimburse most of the drug costs through medical insurance reimbursement to reduce economic burden, greatly enhance patients' burdensomeness and accessibility for drugs, and promote the development of the domestic anti -HIV drug market from the payment side.
Zezhe Pharmaceutical is a pharmaceutical company focusing on tumor, bleeding and hematological diseases, immune inflammatory diseases and hepatobiliary diseases. The company's R & D expenses in the first half of 2022 were RMB 25,537,200, an increase of 29.30%year -on -year; R & D investment accounted for 242.29%of operating income, a year -on -year decrease of 223.43%.
The core product of Zeya Pharmaceuticals Dorfini is the first first -line first -line treatment of advanced liver cell carcinoma to targeting innovative drugs independently developed and listed on Chinese pharmaceutical companies. It officially obtained NMPA approval in June 2021. In August 2022, Donafini tablets are approved for the treatment of progressiveness, local advanced or metastatic radioactive iodine refractory differentiated segmented segments.
In the first half of this year, Zezhe Pharmaceutical achieved operating income of 105 million yuan, an increase of 148.53%year -on -year; net profit of returning to the mother was 247 million yuan, a year -on -year decrease of 39.58%.
The fourth -fourth R & D Junshi creature is an innovative -driven biopharmaceutical company. In the first half of this year, Junshi Biological R & D investment was about 1.062 billion yuan. Test applications are approved by NMPA or FDA. At present, there are three research products in the commercial stage of Junshi creatures, including Tripley Mipide, Etzovicabe, and Adamab.
Tripley Mippy is the core product of Junshi Bio, and it is also the company's first listing product. This product was officially listed and sold at the end of February 2019. It is the first domestic anti-PD-1-1 domestic anti-PD-1 of the State Drug Administration approved to be listed on the market. Single clone antibodies have been approved in China.
Junshi Bio, a small molecule new crown special drug VV116, has entered the later stage of the phase III clinical research stage, and is about to enter the application stage of the drug listing. Junshi Bio -related persons told the reporter of Huaxia Times that from the perspective of the global market, the new crown oral medicine currently approved in the US market, Molnupiravir in Murissa Dong's PAXLOVID in the first half of 2022 is about the global sales of about in the first half of 2022, respectively. $ 4.4 billion and $ 9.6 billion. It can be seen that the new crown small molecules have a huge market demand. In the case of only one domestic new crown small molecule approved, combined with the domestic policies of active prevention of epidemic prevention, VV116 still has greater application value and market potential.
In the first half of this year, Junshi Bio had a net profit of 912 million yuan in net profit. The company said that it was mainly due to the decrease in the company's operating income and the expansion of research and development pipelines and the rapid progress of R & D projects.
Drawing/Wang Yu
Xuexue Editor: Editor of Yan Yuan: Chen Yanpeng
- END -
Sichuan yesterday added 126 new local confirmed cases, and 84 cases of new native non -symptoms were added
At 0-24 on September 12, the latest situation of the new crown pneumonia in Sichuan Province is as follows:126 new local diagnosis cases: 18 cases in Chengdu, 49 cases in Neijiang, 17 cases of Yibin,
"Heart" breakthrough!Kaide's cardiovascular team involved in completing the perfurate

Recently, the cardiovascular team of Cardiovascular Vascular Hospital of Zhuzhou K...