17 batches of medicines are unqualified!Guishan and West Gurise Pharmaceuticals, etc.
Author:Zhongxin Jingwei Time:2022.09.15
Zhongxin Jingwei, September 15th. On the 15th, the State Drug Administration issued 17 batches of medicines that did not meet the requirements.
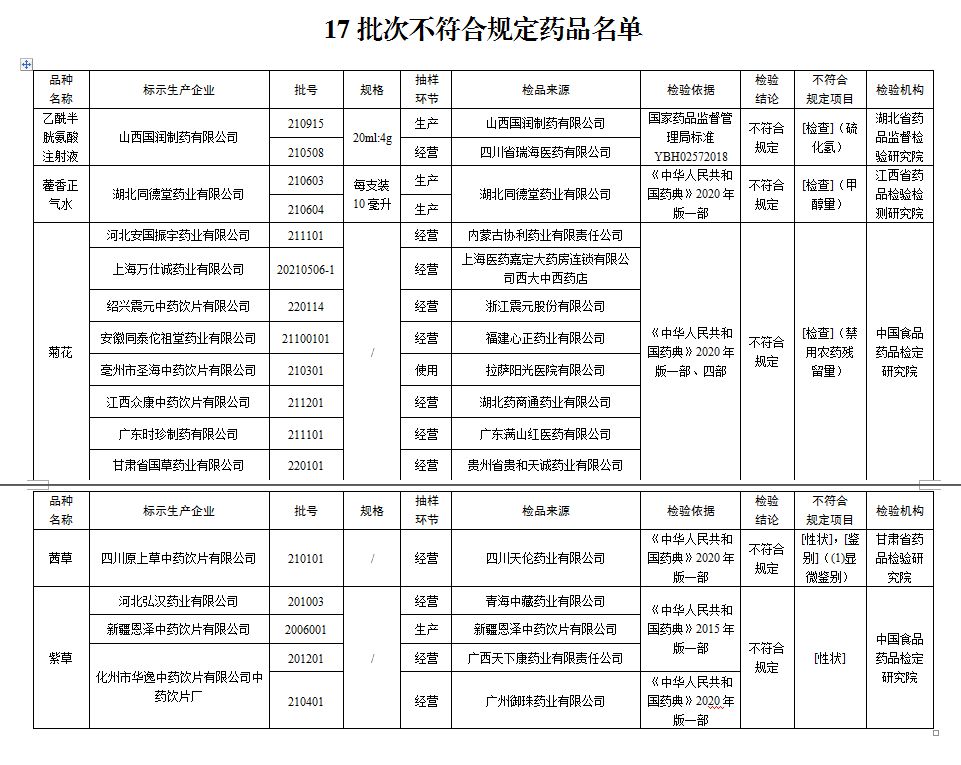
The notice stated that after inspection by 4 pharmaceutical inspection institutions including Hubei Pharmaceutical Supervision and Institute, 17 batches of drugs such as acetylcysteine injections produced by Shanxi Guorun Pharmaceutical Co., Ltd. did not meet the regulations. The relevant situation is notified as follows:
I. After inspection by the Hubei Provincial Institute of Drug Administration, the two batches of acetylcysteine injection produced by Shanxi Gurun Pharmaceutical Co., Ltd. did not meet the regulations and did not meet the requirements of the specified project as hydrogen sulfide.
After inspection by the Jiangxi Provincial Academy of Pharmaceutical Inspection and Inspection, the two batches of Huoxiang Zhengqi water produced by Hubei Tongdutang Pharmaceutical Co., Ltd. did not meet the requirements, and did not meet the requirements of the regulations.
After inspection by the China Food and Drug Inspection Research Institute, it is marked as Hebei Anguo Zhenyu Pharmaceutical Co., Ltd., Shanghai Wanshi Cheng Pharmaceutical Co., Ltd., Shaoxing Zhenyuan Traditional Chinese Medicine Drinking Co., Ltd., Anhui Tongtai Zutang Pharmaceutical Co., Ltd. Shenghai Traditional Chinese Medicine Drinking Co., Ltd., Jiangxi Zhongkang Traditional Chinese Medicine Drinking Co., Ltd., Guangdong Shizhe Pharmaceutical Co., Ltd. and Gansu Guogao Pharmaceutical Co., Ltd. did not meet the regulations, and the project was not in line with the regulations to disable pesticide residues.
After inspection by the Gansu Provincial Academy of Pharmaceutical Inspection and Research, a batch of Qiancao produced by Sichuan Yuancao Traditional Chinese Medicine Drinking Co., Ltd. did not meet the requirements, and did not meet the specified projects including traits and identification.
After inspection by the China Food and Drug Inspection Research Institute, it is marked that 4 batches of authentic purple grass produced by Hebei Honghan Pharmaceutical Co., Ltd., Xinjiang Enze Traditional Chinese Medicine Drinking Co., Ltd. and Huayi Huayi Traditional Chinese Medicine Drinking Factory Co., Ltd. It meets the specified projects as traits.
The notice stated that the drug supervision and management department has required relevant enterprises and units to take risk control measures such as suspension of sales, recalls, and other reasons for the reasons that do not meet the prescribed drugs to conduct investigations and rectification on reasons for reasons that do not meet the requirements.
The State Drug Administration requires relevant provincial drug supervision and management departments to organize investigations on suspected illegal acts existing above -mentioned enterprises and units in accordance with the Drug Administration Law of the People's Republic of China, and publicize the results of the results in accordance with regulations.
Source: Website of the State Drug Administration
(Zhongxin Jingwei APP)
Pay attention to the official WeChat public account of JWVIEW (JWVIEW) to get more elite financial information.
- END -
The 9 -year -old boy suddenly screamed and walked around at night.

The little boy who lives in Shaoyang (pseudonym) is 9 years old. He has no obvious...
Naito County realizes "dynamic clearance"
Source: Naqu Rong MediaAfter the first case of input -type sieve positive 1 case in Suoxian on August 9, Naqu City successively discovered 6 input types of infected infections. Suoxian became the firs