10 batches of medicines are not compliant!Cold particles involved in children, Sichuanbei cough syrup, etc. →
Author:China Economic Network Time:2022.06.21
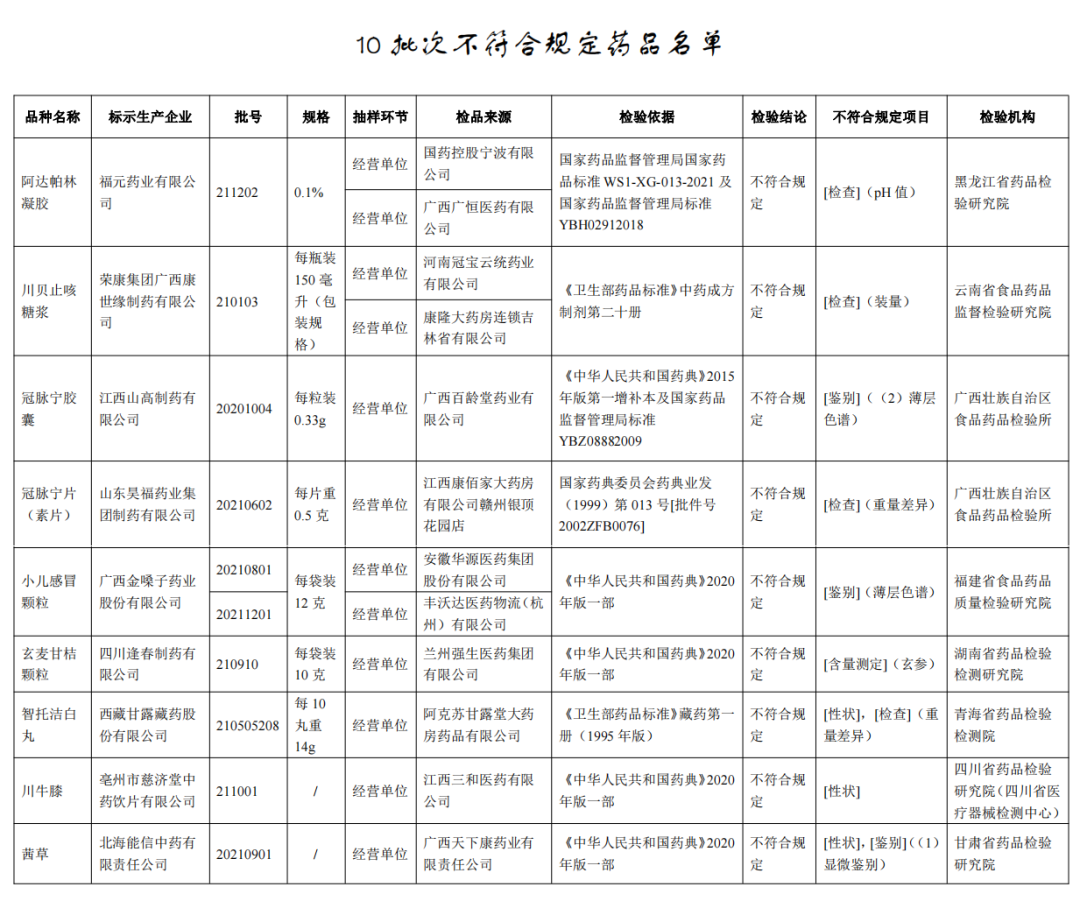
The State Drug Administration recently issued a notice that after 8 pharmaceutical inspection institutions such as Food and Drug Quality Inspection and Research Institute of Food and Drug Quality Inspection of Fujian Province, 10 batches of children's cold particles such as Guangxi Golden Vol. Regulation.
Screenshot of the official website of the State Drug Administration
According to the notice, after inspection by Heilongjiang Pharmaceutical Inspection and Research Institute, a batch of Adapolin gel produced by Fuyuan Pharmaceutical Co., Ltd. does not meet the regulations and does not meet the requirements of the specified project as a pH value. It is reported that the pH value is a hydrogen ion concentration index, which is used as a measuring indicator for the degree of acid and alkali.
After inspection by the Institute of Food and Drug Supervision and Inspection of Yunnan Province, a batch of Chuanbei cough syrup produced by Rongkang Group Guangxi Kangshiyuan Pharmaceutical Co., Ltd. did not meet the requirements and did not meet the requirements of the regulations as the installation. It is reported that the volume is an indicator that reflects the weight or capacity of the medicine. It is suitable for solid, semi -solid, and liquid preparations. It is stipulated that it should be checked in accordance with the lowest amount inspection method. Do not meet the regulations that will lead to insufficient clinical dosage dosage.
After inspection by the Food and Drug Inspection Institute of Guangxi Zhuang Autonomous Region, a batch of coronal pulse capsules produced by Jiangxi Shan Gao Pharmaceutical Co., Ltd. did not meet the requirements and did not meet the requirements of the regulations as a identification; One batch of coronary Ning Ning tablets (vegetarian tablets) does not meet the regulations, and it does not meet the requirements of the specified project as a weight difference. It is reported that the identification items are mainly used to distinguish the characteristics of drugs. The means include micro -identification, spectral identification, etc. The thin layer chromatography is a commonly used method. The weight difference is the indicator of the uniformity of the drug, which is one of the important parameters to ensure accurate administration.
It was inspected by the Food and Drug Quality Inspection and Research Institute in Fujian Province. The two batches of children's cold particles produced by Guangxi Golden Vol.
After inspection by the Hunan Pharmaceutical Inspection and Inspection and Institute, a batch of Xuanmai Ganjia particles produced by Sichuan Fengchun Pharmaceutical Co., Ltd. did not meet the requirements, and did not meet the requirements of the specified project as the content measurement. It is reported that the content measurement system refers to the content of the raw materials and the effective ingredients in the preparation of the effective components in the test method, and chemical, instruments or biological measurement methods can generally be adopted.
After inspection by the Qinghai Pharmaceutical Inspection and Inspection Institute, a batch of Zhito Jiechi White Pills produced by Tibetan Ganlu Tibetan Pharmaceutical Co., Ltd. did not meet the requirements and did not meet the requirements of the specified projects as traits and weight. It is reported that the appearance, odor, taste, solubility, and physical constant are recorded under the traits, which reflect the quality characteristics of the medicine to a certain extent. Traditional Chinese medicines do not meet the regulations, and may involve the deviation of the species of medicinal materials, defects, and improper storage of the processing process.
After inspection by the Sichuan Pharmaceutical Inspection and Research Institute (Sichuan Medical Device Inspection Center), a batch of Sichuan Achyranthes knee produced by Tzuchi Tzuchitang Traditional Chinese Medicine Crimination Co., Ltd. did not meet the regulations and did not meet the requirements of the specified projects as traits.
After inspection by the Gansu Provincial Academy of Pharmaceutical Inspection and Research, a batch of Qiancao produced by Beihai Nengxin Traditional Chinese Medicine Co., Ltd. does not meet the requirements and does not meet the requirements of the specified projects as traits and identification.
The State Drug Administration stated that the drug supervision and management department has required relevant enterprises and units to take risk control measures such as suspension of sales and recalls, and conduct investigations and rectify rectification on the reasons of not compliance with the prescribed reasons.
In addition, the State Drug Administration requires relevant provincial drug supervision and management departments to organize investigations on suspected illegal acts existing above -mentioned enterprises and units in accordance with the "Drug Administration Law of the People's Republic of China", and publicize the results in accordance with regulations.
- END -
There are 5 new local infections in Beijing, and the high risk is upgraded at 1 place
Zhongxin Jingwei, June 21st. Healthy Beijing WeChat News, on June 21, Beijing held a press conference on the 372th new crown pneumonia epidemic prevention and control work. Liu Xiaofeng, deputy dire
The first new type of left -hearted ear seal blocker implantation was successfully launched in Wuhan University People's Hospital

Wuhan Evening News, June 27 (Correspondent Yang Cen Confucian) On June 21, Profess...