How can the new drug clinical trials restrict the Chinese pharmaceutical industry?
Author:China News Weekly Time:2022.09.12

not
When domestic clinical trials accompanied by the development of China's pharmaceutical industry
When singing all the way
Industry insiders pointed out
There is still a lack of innovation behind it
Low standards, insufficient time for researchers, etc.

In the development of the most active new drug in China, the development of anti -tumor drugs, clinical trials are concentrated in the four major medical institutions, namely the Cancer Hospital of the Chinese Academy of Medical Sciences, the Beijing University Cancer Hospital, Fudan University Cancer Hospital, and the Cancer Hospital of Sun Yat -sen University. In June, a CRO company (contract R & D service organization) undertook the clinical trial of pharmaceutical companies conducted research in one of the four institutions. The person in charge of the CRO company told "China News Weekly" that under the hands of a well -known expert, there are more than a dozen new drug clinical trial projects such as melanoma. In the project.
In the past ten years, especially by the reform of the 2015 drug review, China Innovation Pharmaceutical has entered the fast lane. From 2010 to 2020, the number of clinical trials received by the State Drug Administration (hereinafter referred to as the "State Drug Administration") increased from 29 to 428 in 2020, with an average annual growth rate of 32 %.
Clinical trials are one of the clinical research. The purpose is to determine the efficacy and safety of the drug. It is a key step in the approval of new drugs. The quality of the clinical trials of new drugs is related to the life and death of pharmaceutical companies in business competition, which also affects the accessibility of drugs. It is also the key to whether China can become an innovative drug.
Insiders pointed out that when domestic clinical trials accompanied by the development of China's pharmaceutical industry, behind the highlights of the division of labor, there are also problems such as insufficient innovation, low standards, and insufficient time for researchers.
The executive meeting of the State Council held on June 29 proposed that some high -level hospitals should be selected to carry out pilots to improve clinical research and achievement transformation capabilities and promote the improvement of medical and health services.
Tests after the reform of drug review are irregular issues
According to the "Annual Report of the Progress of China's New Pharmaceutical Registration Clinical Test Progress (2021)" released by the State Drug Administration (CDE) in April this year, the number of Chinese drug clinical trials in 2021 exceeded 3,000 items. Among them, the number of clinical trials of new drugs was 20,33, an increase of 38.0 % from 2020. In 2021, the clinical trials of Chinese drugs are still mainly chemical drugs, accounting for 70.8 %; followed by biological products, 26.7 %; the least Chinese medicine, only 2.4 %.
China's clinical trials are in a state of blowout. In the Junior Inspection Company of the Clinical Test of Beijing Dongcheng District, the founder Cai Xuliu told "China News Weekly" that in recent years, the business volume has shown explosive growth. Line for about three months. This is completely different from the pre -2015 image.
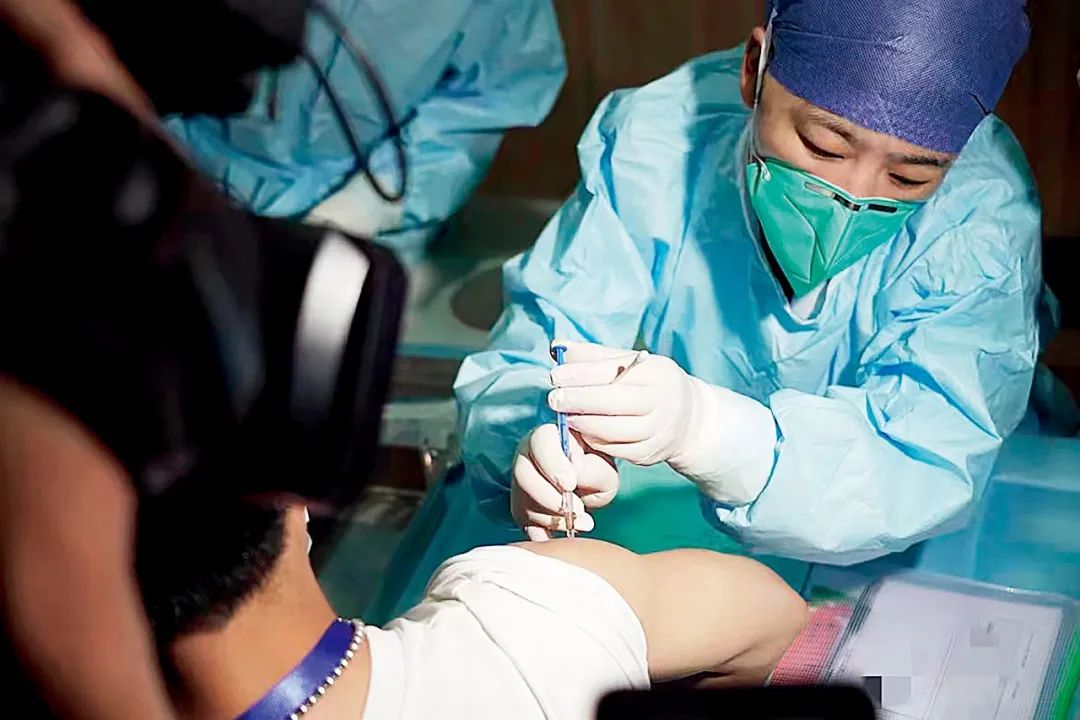
On May 1st, a clinical trial volunteer injected the new crown virus in Hangzhou, Hangzhou, Zhejiang. Picture/Xinhua
Drug clinical trials are generally divided into I, II, III, IV, and pharmaceutical biological equivalent tests. They are funded by pharmaceutical factories and carried out in medical institutions. Clinical research supervisors will also be hired to supervise. Some pharmaceutical companies that want to be better in clinical trials can also ask third -party audit companies to check the data of clinical trials.
In 2009, Cai Xu Liu established the earliest domestic clinical trial third -party audit company. However, at that time, there were few demands in China, and few pharmaceutical companies were paying attention to the quality of clinical trials.
Mao Yimin, chief physician of the Department of Gastroenterology, Affiliated to the School of Medicine of Shanghai Jiaotong University, is the earliest group of doctors in China to conduct clinical trials in China. So far, it has nearly 30 years of clinical trial experience and worked in CDE. More than ten years ago, when he went abroad to participate in some conferences to introduce the development of Chinese clinical trials, participants from Western countries often showed an unbelievable expression: at that time, the clinical trials of China were mainly initiated by the pharmaceutical factory. Data may have problems due to conflict of interests, data quality, normative, and even authenticity.
On July 22, 2015, the State Food and Drug Administration issued the "Announcement on the Inspection of Pharmaceutical Clinical Test Data Research". Under the strong supervision of the "7 · 22 storm", now the industry practitioners generally believe that there are almost few clinical trials that there are real problems with authenticity, because data fraud is an absolute red line, but the irregular problems of clinical trials are not standardized. But it needs to be resolved.
The clinical trial management specifications (GCP) system was born in the United States, which is the leader of the pharmaceutical industry. The US Food and Drug Administration (FDA) promulgated GCP in 1981. Since then, the European Union, Japan and other countries have followed up to formulate GCPs with their own characteristics. In March 1998, the Ministry of Health of China promulgated the first edition of the "Pharmaceutical Clinical Test Management Specification GCP" (trial).
The new version of the GCP required by July 1, 2020, researchers should ensure that all clinical trial data are obtained from clinical trial source documents and test records, and are accurate, complete, readable and timely. Source data should have a series of characteristics such as attractiveness, readability, similarities, and primitive.
The State Drug Administration conducted clinical trial supervision and random investigation of the 10 medical device registration application projects in November 2020, involving 27 clinical trial institutions. The key to find out that the key to Anxu's creatures is to research products. A kit used to detect HIV, hepatitis C virus, hepatitis B virus, and syphilis antibody has unsatisfactory problems in clinical trials. According to reports, the product was conducted in clinical trials in the First Affiliated Hospital of Zhejiang University Medical College. The electronic photo shooting time, place and the actual time of clinical trials of medical institutions were inconsistent with the actual time of clinical trials. Data reliability is greatly reduced. In 2021, the State Drug Administration decided not to register for the product.
In February 2021, the "British Medical Magazine" published an article from the School of Pharmacy of Johns Hopkins University. Researchers found that since 2008, there are still a large number of clinical trials in mainland China. The effect of statins for patients with coronary heart disease. He was recommended as a first -line medication for patients with coronary heart disease more than ten years ago as early as ten years ago. But for more than ten years, China has explored the endless clinical research of Batin's necessary clinical research on whether such patients have.
In this regard, Zhai Xiaomei, Executive Director of the Life Ethics Research Center of the Chinese Academy of Medical Sciences, explained to China News Weekly that in the case of a safe and effective drug solution, only two valid schemes can be compared with comparison. Involved the placebo, that is, a comparative test of invalid therapy. Zhai Xiaomei analyzed that there are many domestic clinical trial institutions in China, but their ethical review committees have uneven development. For example, in the protection of subjects, many ethical censorship simply equals its consent and pays too much attention to procedure compliance. However The substantial judgment of ethical protection, including a study of the benefits and risks of a study to the subject, whether a clinical trial has scientific value, etc.
"Outsourcing" clinical trial
The irregularities of clinical trials are related to the good and bad of employees.
In 1996, when Mao Yimin was still a resident and began to participate in clinical trials, almost all jobs had to do doctors and nurses to complete. At that time, there was no division of labor such as clinical research supervisors (CRA) and clinical research coordinator (CRC). Simply put, the role of these positions is to assist medical care to complete clinical trials. Essence
On behalf of the applicants of clinical research supervisors, the interests of the applicant, is the interests of the pharmaceutical company, and is responsible for follow -up and regularly monitor the development of clinical trial projects in the hospital. Some large pharmaceutical companies choose to form their own clinical teams, including their own CRA, and some companies outsourcing clinical trial work to CROs, and CROs send CRA. CRO is the contract research organization to take responsibility for choosing hospitals, doctors, clinical trial supervision and other work for pharmaceutical companies. It is also responsible for data collection management, statistical analysis, and report writing. The business almost covers the entire process of new drug research and development.
On April 24, 2018, a chemical and drug innovation center in Lianyungang, Jiangsu, the staff of the clinical test center checked the label information. Figure/IC

Clinical research coordinator, mainly helps researchers to complete the auxiliary work such as the subject contact, registration, and data entry of the subject. The ability and professional background for their required and professional background are equivalent to doctors assistant. Most of them are sent by SMO (clinical trials on -site management organization). In the 1970s, the CRO industry accompanied the complexity of American drugs and the intensity of competition in the pharmaceutical industry. In China, CRO is an emerging industry that has only developed in the past two decades.
Before the strictest data verification incident in 2015, some CRO industry executives bluntly stated in an interview that due to the lack of regulatory systems, CROs are mixed with fish and dragons, and they are competing at low prices. After the "7 · 22 storm", the industry ushered in shuffling. Zhang Dan, co -founder of Global Clinical CRO Kunyu Company, told China News Weekly that after 2015, the industry was more centralized, small companies were eliminated, and CRO companies with high -quality clinical trials won rapid development.
The efficiency brought by this division of labor is necessary. Mao Yimin introduced that because clinical trials are a team cooperation, it involves all aspects and very detailed, and it is difficult for doctors in the research team to complete the task. Moreover, clinical trials are not compulsory evaluation content, and more voluntary participation. But the problem is that CRA and CRC have been flowing too much. Depending on the approval time, the subjects of the subjects, fast and slow, trial drugs, etc., the clinical test items are short for several months and long for several years, but the CRC and CRA may have changed. Big, this also makes it difficult for researchers to grasp all details.
In 2020, after graduating from Fudan University, Zhang Jiaqi, a major of pharmacy, joined a multinational pharmaceutical company and became one of the few CRA recruited by the school. She originally thought that such large enterprises would have standardized training. However, the reality was that she was "thrown" directly into the project, and at the same time was responsible for the supervision of 7 clinical trial projects.
"The industry is too impetuous." A senior industry person said that on the one hand, because of the large talent gap in the industry, it makes it easier for employees to change jobs; on the other hand, because of the serious loss of personnel, pharmaceutical companies, SMO, and CROs will not be Willing to invest in training, causing a vicious circle.
Last year, Zhang Jiaqi joined a small overseas pharmaceutical company. The clinical trial work hired CRO. Although the other party is a domestic header, its CRA level is not stable. She said that many data in clinical trials have only one chance to record. There is a word in this industry that "without records without records." However, because of the irresponsibility of the CRA work in cooperation, the data is missing, and some test results are contradictory. Some of the information that should be handed in at a certain point of time has not been handled, resulting in a loss of losses that pharmaceutical companies are recovered. This also means that the process of drug development is slow. At the same time, in the participation of multiple parties, there is a problem of conflict of interest. According to industry insiders, in general, a clinical trial project contract of tens of millions of yuan in pharmaceutical companies basically pays 1/3 of the research costs of the hospital, CRO's service fee accounts for 1/3, and the other 1/3 is paid to the data Statistics analysis company, SMO company, etc.
The CRC sent by SMO was originally served by researchers, but was funded by pharmaceutical companies. Former Deputy Director Xu Juncai, former deputy director of the Shanghai Medical Clinical Research Center, previously wrote that foreign research assistants are more like a member of the experimental team, not employees provided by third -party companies such as SMO. Sometimes SMO companies directly serve the applicants to generate data. Not objective. Professor Wu Yilong, a professor at the Guangdong Institute of Lung Cancer, also mentioned earlier this year that some CRA may "forced" researchers to change their side effects out of pressure.
The sought -after experts and their scarce time
In 2019, Xue Quan, the deputy general manager of CRO company Beijing Nuo and Demei, accompanied a foreign counterpart to the clinical research center for project inspection. Although he agreed with the researchers to meet at 11:30 noon in advance, after arriving at the research center It hasn't ended until more than 12 o'clock that it had only done more than ten minutes of exchanges in the research project in the doctor's office. When the company's CRA works in the hospital, it usually only uses the time of the researchers to check to check the original data with the doctor.
Peng Zhen and others of Anhui Provincial Hospital analyzed 32 clinical trials carried out by a medical institution after "July 22" and found that there were 387 unqualified points in 29 projects. The study was published in March last year in "China Hospital Pharmacy Magazine. Specifically, the most problems are the traceability of the clinical trial records and clinical examinations, testing and other data. There are 270 unqualified items, accounting for 69.77 %. The completeness of the data chain, the collection, preservation, transportation and transfer records of clinical trials.
The study found that there were many problems in the clinical trial process, and the main reason was that the test did not receive the attention of researchers. In addition to busy medical work, researchers participate in clinical trials, and use more time to use their spare time. This is consistent with Cai Xuliu's observation. After the company has undertaken the on -site audit of nearly 1,000 clinical trial projects in recent years, he felt that the root cause of many problems lies in the lack of energy of researchers.
According to the latest edition of the latest version of the "Quality Management of Drug Clinical Test", the main researchers (PI) must ensure that all personnel participating in clinical trials fully understand the test schemes and test drugs, and clearly make their own division of labor and responsibilities in the experiment to ensure the clinical clinical The test data is true, complete and accurate. The main researchers are the controller of the quality of clinical trials who need to be inspected by the applicants and regulators unconditionally.
However, Cai Xu Liu pointed out that because the doctor as a researcher was too busy, many of the work that should have been responsible for the doctor, including informed consent, medical judgment, medical treatment, medical history records, etc., all handed over to CRC. Xu Juncai pointed out in the article that many researchers have highly dependent research assistants, forming a data production chain, and even the subjects such as screening and studying medical records involved in professional medical tasks have also been transferred to CRC's head.
At the end of November 2019, the State Health and Health Commission and the State Drug Administration jointly issued the "Provisions on the Management of the Drug Clinical Test Institution". The institutions that can carry out clinical trials have been changed from qualification certification to filing management, which greatly simplifies the institution application process. It is believed that this will make the management concept of clinical trials from the previous "strict advancement" and gradually transition to the "wide entry and strict" model, which also increases the higher proportion of domestic clinical trial institutions. As of the end of 2019, 839 clinical trial institutions across the country have filed. By the end of 2021, a total of 1,075 drug clinical trial institutions in the country increased by 28.1 %.
Several industry people believe that letting go is a good thing, because this greatly alleviates the tension of clinical research centers and researchers, and pharmaceutical companies and CRO companies have more choices. On the other hand, the system of Chinese pharmaceutical listing permit holders ensures that whether it is the clinical trial of the drug or the outsourcing of the outsourcing, and in the end, they must be held accountable to the listing license holder. Essence
However, the current situation of unequal regional distribution of medical resources in China, clinical trials are still highly concentrated in some regions and well -known hospitals. According to CDE's 20121 report, in the past three years, the leaders and participants of the clinical trial team are still mainly Beijing, Shanghai, Jiangsu, Guangdong, etc. The well -known GCP institutions and well -known professors in the industry have become the targets of pharmaceutical companies, and participants from all parties are entangled in the departments of these well -known researchers.
According to the CDE report, in 2021, the top 5 provinces and cities of the clinical trial team leader did not exceed 60 % within 6 months. According to the CDE "Pharmaceutical Clinical Test Registration and Public Announcement Platform", the three institutions with the largest number of newly undertake registered drugs in 2021 are: West China Hospital of Sichuan University, First Hospital of Jilin University, Henan Cancer Hospital, the clinical clinical The number of trials exceeds 300 cases, that is, these hospitals will undertake a clinical trial almost every day. According to the statistics of this platform, the number of clinical trials undertaken by a well -known tumor expert in 2021 is close to hundreds. Professor Wu Yilong, a professor at the Guangdong Institute of Lung Cancer, also expressed his concerns earlier this year. "Domestic researchers take the lead in multiple clinical trials at the same time to ensure fairness? How to ensure that sufficient energy takes into account these projects at the same time?"
"In the past, scientific and detailed clinical scientific research has become a pilot data production market with many division of labor today." Xu Juncai pointed out that the core of clinical trials was based on the scientific medical judgment of researchers. These observations and discovery are the most important original data in clinical trials. If these data are not from professional researchers, clinical trials will eventually only focus on processes and ignore the nature of science.
Xu Juncai analyzed that today's practitioners should seriously think about what is really convincing when they really do a brand new new drug clinical trial at home and abroad. Many medical information involving the subjects, new and unknown risks, are more dependent on researchers than general clinical diagnosis and treatment to judge. Simple CRA+CRC+researcher signing model is not a scientific clinical trial.
How to improve?
In Cai Xuliu's view, clinical trial errors are almost inevitable, because such research rely on people and difficult to standardize. In this field, there is a sentence that "never perfect clinical trials." However, this does not mean that China's clinical trials have no institutional improvement space.
Li Yanran is a senior practitioner in the American biotechnology and CRO field, and has many years of experience in the development and clinical trial management of new drugs. She told "China News Weekly" that American doctors, especially well -known doctors, are also very busy. Pharmaceutical companies and CROs must also work hard to maintain their relationship with researchers so that clinical trial projects can get more attention. However, there are also approach to balance between the two sides. CRO is familiar with experts in various diseases, and there is a system that scores the main researcher. This score is the basis for selecting researchers for pharmaceutical companies, and also determines that researchers can get compensation. According to a domestic CRO executive, large domestic CRO companies generally have their own assessment forms, but when choosing PI, they are also subject to more doctors and limited funds. They do not rely on evaluation.
How to improve the enthusiasm of researchers to participate in clinical trials and ensure the quality of the experiment, Mao Yimin pointed out that a set of incentives and punishment mechanisms should be established to add researchers participating in clinical trial projects and can complete high quality. Division; otherwise, it should be restricted or even suspended. Researchers who have authenticity problems in data establish a blacklist system. In this way, the researchers will be cautious to undertake the quality of the project and ensure the quality of the project.
In addition to doctors, there are two institutions involving clinical trials in domestic medical institutions: ethical committees and GCP institutions, responsible for the establishment of clinical trials, signing contracts, quality control of clinical trials, test questions, etc. However, Mao Yimin pointed out that most of the domestic GCP institutions in China are very limited. Some come from the pharmacy department, and some come from the scientific research department. Moreover, a considerable proportion of personnel have no experience in clinical trials, because some GCP institutions cannot be promoted according to the doctor's system, nor can they be promoted according to the scientific researcher's system. Essence This has made domestic clinical trials more pursuing formal norms, ignoring the nature of clinical trials and innovation of high -efficiency work.
In Europe and the United States, the person in charge of a multinational CRO company introduced that what clinical trial projects and how to carry out are all researchers themselves. If the test is not well developed, it is difficult for pharmaceutical companies to work with them.
The relevant documents related to the reform of the drug review in 2015 will be adjusted from the "medicines that have not been listed in China" at that time to "medicines that have not been sold at home and abroad in China", which greatly changes the definition of the drug "new". Essence Xue Quan told "China News Weekly" that before 2015 and even in 2017, the so -called innovative drugs in China were actually new drugs that were quoted, because many of them were already listed abroad. When clinical trials were carried out, most of the solutions were copied. In recent years, some innovative medicines with real new targets have appeared in China, which requires doctors to invest more energy and improve the level of research.
In the United States, Li Yanran said that clinical trial practitioners are following the development of new drug development, the use of new technologies, and changes in new environments. For example, the new crowns have been popular in the past two or three years, because of the limited flow of personnel, data collection at the clinical research center has become difficult. As a result, researchers used technology to carry out remote tracking and monitoring. On the other hand, if pharmaceutical companies want to win in the competition, they must rely on new ideas and technologies, such as developing gene therapy methods, and CRO must keep up with the cutting -edge dynamics of the pharmaceutical factory and recruit talents in related fields. On the other hand, Zhang Dan, co -founder of Global Clinical CRO Kunyu Company, pointed out that among the 1075 medical institutions that can carry out clinical trials in China, only 100 have carried out generic drugs, innovative drugs, and international multi -center clinical trials at the same time. Therefore, although China is the country with the most patients in the world, there is a considerable gap between the design and leadership of the international multi -center clinical trials. Most doctors are unable to design clinical trials that meet international standards.
Li Yanran said that in the United States, whether it is a pharmaceutical company, researcher or CRO company, the important reason for ensuring that the parties can be compliant is that the supervision is very strict and the cost of making mistakes is high.
As early as 1977, the FDA set up a biological research test and inspection system to conduct a comprehensive examination of the clinical trials of drugs to ensure the authenticity, integrity, and reliability of clinical research data. Because of the late start, the Chinese State Drug Administration still has a large gap in the size of the inspectors compared with the FDA of the United States.
When the State Drug Administration's Pharmaceutical Audit Center mentioned that when the personnel were expanded to 1600 in 2020, the same period, the total of more than 7,000 FDA drug reviewers in the United States, of which the drug review and research center responsible for new drugs and generic drug reviews About 4600 people, huge reviewers and inspectors are the cornerstone to support the FDA drug supervision system. According to Li Yanran, it is important that because the FDA will be inspected from time to time, clinical trial applicants must be prepared at any time. CRO will send experienced and familiar personnel to assist pharmaceutical companies to eliminate risks.
Because of the relatively mature legal system and regulatory system, the risk of corporate fraud is high, and it pays more attention to quality management. Cai Xuliu said that the size of the US drug clinical trial third -party audit company is relatively small. On the one hand, many pharmaceutical companies in China are scattered and lacking mature talents and experience; on the other hand, it is also in the outbreak period of innovative drug research and development, so more market audit services are needed as supplements.
Repeat applications and targets,
What is the irrational of clinical trials?
Today, in Maryland, the US where FDA is located, more and more Chinese innovative pharmaceutical companies who are preparing to go to sea have set up offices nearby to learn the review rules of the US FDA, which represent the highest standards in the field of biopharmaceuticals. In 2018, the State Drug Administration was elected as a member of the ICH (International Human Pharmaceutical Registration Technology Coordination Association) Management Committee, which means that the new drug clinical research standards are formally connected with international standards. Join the WTO (WTO) this time.
At a meeting last year, Zhou Siyuan, deputy director of the drug review center of the State Drug Administration, pointed out that in the case of surging quantity, China's drug review and approval still did not get rid of the various problems brought by repeated applications. Among the 39 innovative drug approved by the National Drug Administration of China from 2018 to 2020, the First-in-Class (completely innovative) new drugs, accounting for 5 %. At the same time, of the 160 innovative drugs approved by the FDA, 60 FIRST-IN-CLASS drugs were reached, accounting for 38 %. In addition to the proportion of new drugs for anti -tumor drugs, antivirals and antivirals and analgesic drugs to the FDA approval, there are fewer other types of new drugs, and the problem of homogeneity of products is more prominent. Just as the industry has hotly debated PD-1 monoclonal antibodies, there are 276 registered acceptance numbers and 42 enterprises, which greatly wastes approval resources and clinical research resources.
Richard Pazdur, director of the FDA Tumor Excellence Center, published an article in the "New England Medicine" magazine in December 2021 to criticize the phenomenon of such drugs. Although the FDA has approved 7 antibodies for the PD-1/PD-L1 channel, covering more than 85 indications, there are still more than 2,000 clinical trials. Reduration of such drugs. A senior person in the pharmaceutical industry told China News Weekly that even if PD-1 drug profit space is already very low and cannot be approved to be listed, Chinese pharmaceutical companies still enter one after another. One reason is that these pharmaceutical companies have aimed at policy policies dividend.
In 2008, the "major new drug creation" led by the Ministry of Science and Technology, the Development and Reform Commission, and the Ministry of Finance's "major new drug creation" was launched by major national science and technology. It aims to promote the research and development of China's new drugs from imitation to innovation. By the end of 2020, the "major new medicine creation" specially supported more than 3,000 topics, and the central government invested 23.3 billion yuan, which is an important measure in the history of China's innovative drug development.
There are hundreds of thousands of biomedical industry parks in China. With the support of the policy, the aforementioned pharmaceutical industry explained that, especially in second- and third-tier cities, companies developing PD-1 in these parks may be approved for production land and can be supported by tax reduction and exemption. Therefore Essence Pharmaceutical companies prefer to develop PD-1 drugs because it belongs to innovative drugs, but it is not too "innovative", that is, only a little change or exploring some new indications for targets. If there are no new drugs that have not been approved abroad, it is difficult for officials to attract investment. A manager who once worked as a manager in a national pharmaceutical high-tech industrial development zone in a province revealed to China News Weekly that around 2020, there are more than 100 companies that develop PD-1 in the industrial park. Generally speaking According to the progress of clinical trials, government supporting funds ranging from one million to 10 million yuan.
A large number of biotechnology companies were listed, and capital poured in for a while. Some insiders analyzed that capital can make a lot of money after the capital packaging enterprise is used to enable it with a higher market value. In this process, whether the new drug is really valuable, and even whether it can be approved to go public is no longer important. This has spawned a number of repetitive and low -level research projects.
A person who once worked in CDE told China News Weekly that they sometimes communicated with pharmaceutical companies that some drugs were not worthy of clinical trials for repeated research. Pharmaceutical companies still insist on starting, because they have taken the money of investors. Even if they fail the final research and development, they can also be blamed on "investment in investment."
However, related changes are happening. In July 2021, the Drug Review Center of the State Drug Administration announced the "Principles of the Clinical R & D Guidance of Anti -tumor Drugs with Clinical Value", emphasizing the development of new drugs with clinical value, opposing low -level repetitive construction, and becoming the industry in the industry. Important signal.
Zhou Siyuan said that his institutions will follow the requirements of more adaptation to the laws of drug innovation and development and reflect clinical value. In particular, the clinical value exploration of the current existence of drug innovation is insufficient, and the clinical value of low standard assessment is adopted, and the basis of clinical value assessment is unscientific and inadequate issues, and the research and development and review standards are established as soon as possible. Through the adjustment of the drug review center, it has promoted China's drug innovation to truly become an internationally recognized "global new", from quantitative to qualitative changes.
(Zhang Jiaqi in the article is a pseudonym, and the intern Du Yi also contributed to this article)
Send 2022.9.12 Total Issue 1060 "China News Weekly" magazine
Magazine Title: How to restrict China's pharmaceutical industry?
Reporter: [email protected])
Edit: Du Wei
Operation editor: Wang Lin
- END -
Daily meals | Bake a small cake at home, delicious and healthy

Team medical mentor:Professor Yang Zhimin, a well -known health expert, a famous C...
The 14 signals of the body must be paid attention to, some are early cancer signals!
The path of cure of cancer still depends on early prevention, early detection, ear...