Digital Medical Weekly | The State Drug Administration held a chamber of commerce in the management risk of medical device network transaction management; Hongtong Industry received 586 million yuan in financing
Author:Zero One Finance Time:2022.06.20

Produced | Zero One Think Tank
Author | Wang Jingyu, Zhang Zhuojun
Catalog I. First -level market: 12 digital medical companies 'equity financing of 1.036 billion yuan Two and secondary markets: 45 companies' stock prices rose; about 200 million shares of 100 grams of biological shares will be lifted (1) stock price performance (2) Company Dynamic III: Industry Observation: Fujian Provincial Pharmaceutical Supervision Bureau promotes the construction of the information traceability system of traditional Chinese medicine drinking tablets; 1: Appendix 2: Appendix 2: 2022/6/10-2022/6/17) Appendix 2: Digital Medical Enterprise Stock Volatility List (2022/6/10-2022/6/17) Statistics
1. First -level market: 12 digital medical companies' equity financing of 1.036 billion yuan
According to the incomplete statistics of the Zero-One think tank, last week (June 13, 2022-June 19, 2022) in domestic and digital medical-related equity financing events was 12, and the total financing disclosed was about 1.036 billion yuan.
The financing rounds are mainly concentrated in strategic financing, a total of 9. The financing of 1-1 billion yuan reached 3, and the largest financing amount was "Hongtong Industrial" for medical health and medical device research and developer "Hongtong Industrial". It is worth noting that the early entrepreneurial AI pharmaceutical enterprise "Merida Bio", which was established in 2021, received 100 million yuan of seed wheel financing. The company is committed to all kinds of chronic diseases caused by the disorder of the development of immune systems and metabolic systems, including autoimmune diseases, including autoimmune diseases. Various chronic diseases caused by cancer, metabolic diseases and aging.
Table 1: Summary of equity financing information in the digital medical industry
(2022/6/13-2022/6/19)
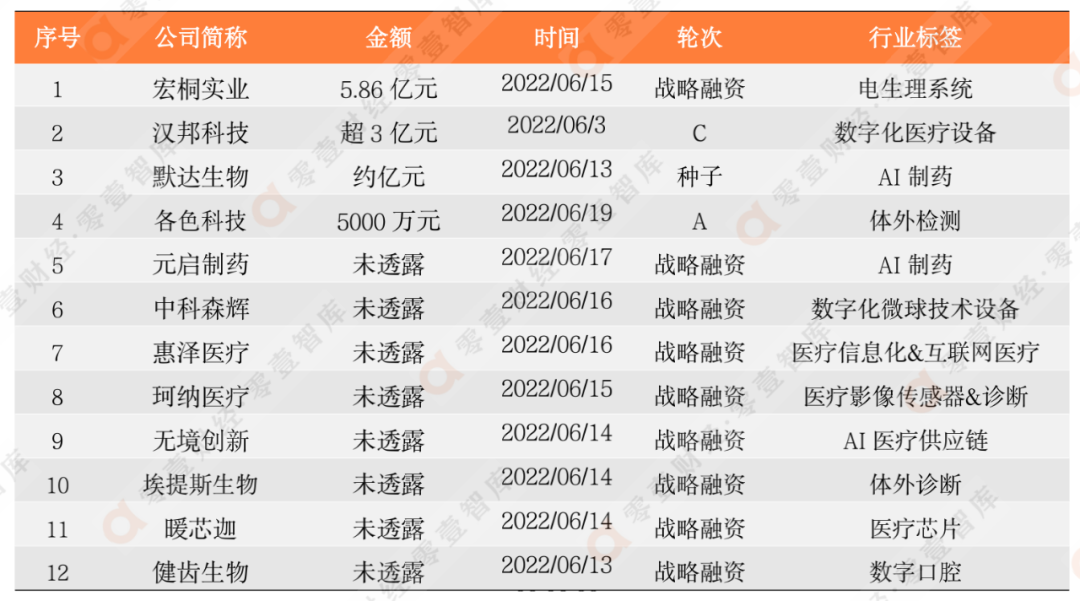
Data source: Zero One Think Tank
Two and secondary markets: 45 companies' stock price rises; about 200 million shares of Bio biological shares will be lifted to lift the ban
(1) Stock price performance
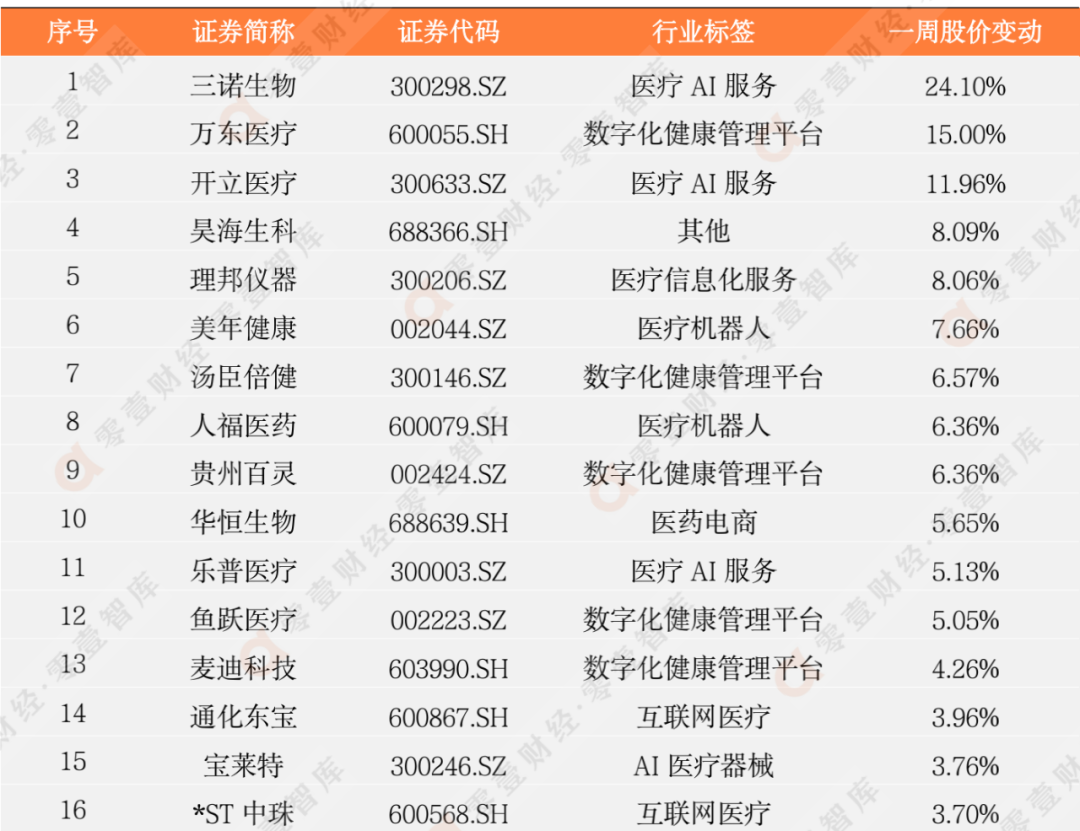
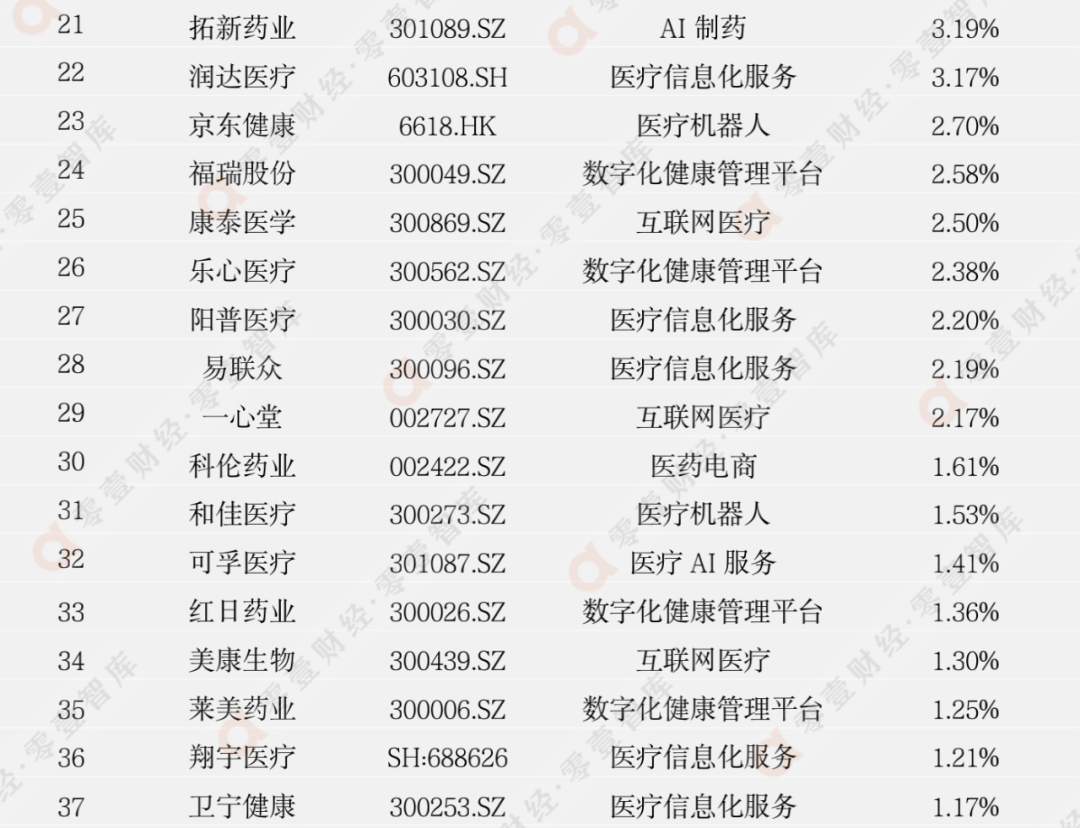
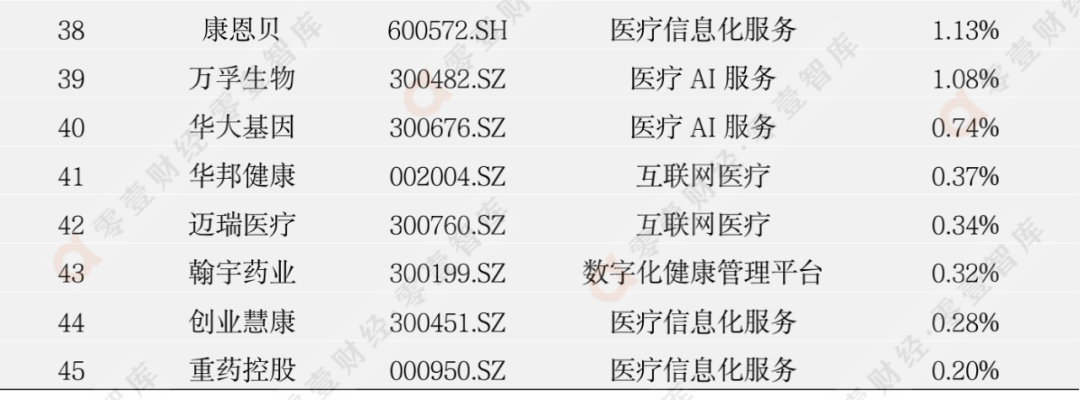
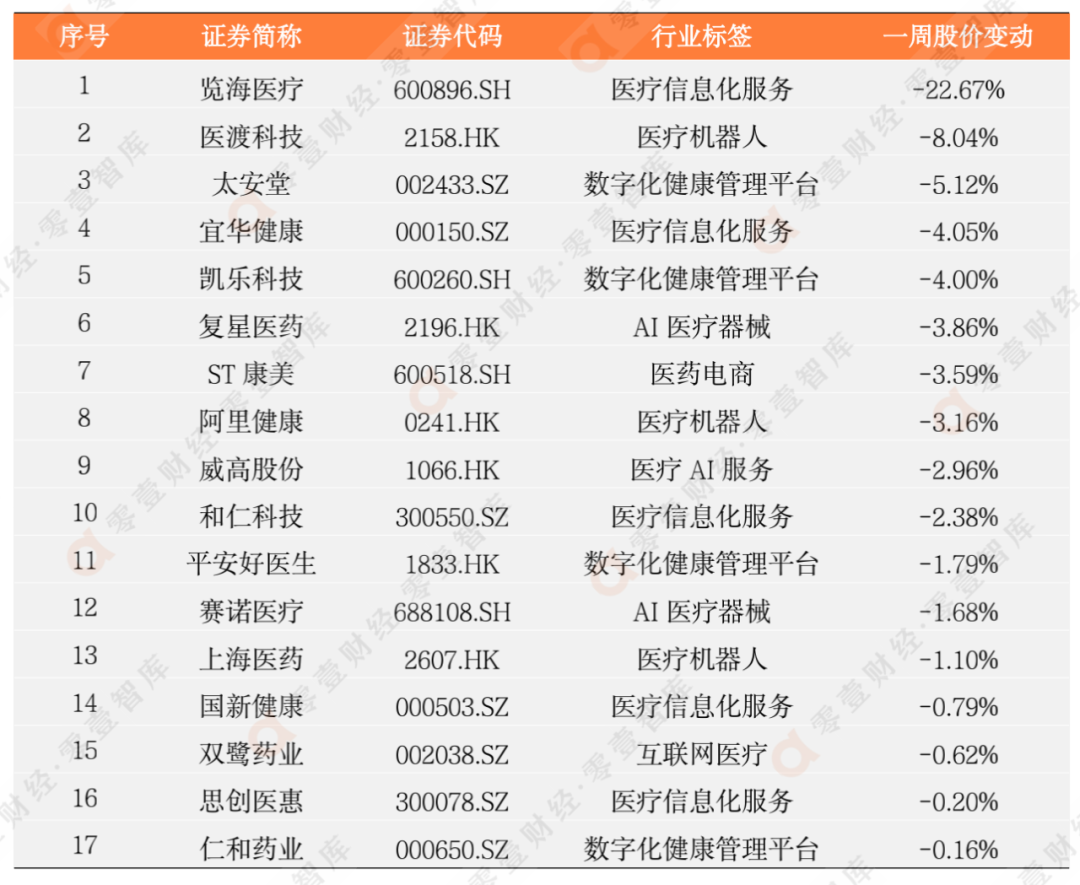
According to the incomplete statistics of the Zero 1 think tank, there are currently more than 100 digital medical listed companies in my country. This report eliminates the company with poor scientific and technological attributes, and has selected 62 companies to observe its secondary market stock price dynamics (see Appendix 1 and Appendix 2 for details).
Last week, 45 listed companies in the digital medical sector rose, and the largest increase was Sannuo Bio (300298.SZ), an increase of 24.10%; 17 companies' stock prices fell, and the largest declines were Lanhai Medical (600896.SH). The decline is -22.67%.
(2) Company dynamics
1. About 200 million shares of a hundred -grams of biology will be lifted
On June 17, the vaccine research and developer 100 grams of creature (688276.SH) released the first public offering of some public offer sales and circulation announcements. The sales period is 12 months from the date of listing of the company. The number of strategic allocation shares of the listing and circulation is 6.6055 million shares, and the sales period is 12 months. The company confirmed that the number of listed circulation is the number of strategic allocation shares at the limited sales period. In addition to strategic allocation of shares, the number of restricted shares listed on the market was 194 million shares. The listing date was June 27, 2022. (Source: 100 grams of creature)
2. The IPO of the Xinghao Pharmaceutical Bei Stock Exchange is accepted, and the fundraising will be used for innovative pharmaceutical industrialization sharing platforms, etc.
On June 17, the new information development of the CMC/CMO platform Xinghao Pharmaceutical (430017.NQ) was accepted. Prior to the fundraising funds in this distribution, the company will temporarily invest funds by self -raising according to the actual situation of the project. After this issuance of raised funds is in place, they will be replaced. After the company's public offering, the issuance result can fully meet the uncertainty of the above listing conditions. (Source: Capital State)
3. Sinopharm Group plans to invest 25 billion yuan in R & D expenses in 5 years to build a three -dimensional pharmaceutical technology research and development system
On June 16, Sinopharm Group (600511.SH) stated that it would further increase scientific and technological innovation. It plans to invest 25 billion yuan in research and development expenses in 5 years to build a three -dimensional pharmaceutical technology research and development system. The Sinopharm Group will focus on the cultivation and strengthening corporate innovation platforms, creating original technical sources, accelerating key core technology research, strengthening innovation layout and transformation of results. Promote the application of scientific research and the development of the industry, promote the deep integration of the innovative chain industry chain of the pharmaceutical industry, and create a world -class comprehensive pharmaceutical health industry group with excellent global competitiveness. (Source: Chinese Medicine Group Science and Technology Innovation Conference)
3. Industry observation: Fujian Provincial Drug Administration promotes the construction of the information traceability system for the informatization system of traditional Chinese medicine decoction;
(1) Policy specifications
1. The Ministry of Finance issued the "Notice of the National Health and Health Commission of the Ministry of Finance on issuing 2022 medical services and security capabilities (comprehensive reform of public hospitals) for subsidy funds (second batch) budget"
On June 14, the Department of Social Security of the Ministry of Finance issued the "Notice of the National Health and Health Commission of the Ministry of Finance on issuing 2022 medical services and security capabilities (comprehensive reform of public hospitals) for subsidy funds (second batch) budget" (hereinafter referred to as Notice). The second batch of financial subsidy funds has a total of 3 billion yuan, which will be directed to 15 places such as Taiyuan, Shanxi Taiyuan, Hohhot, Inner Mongolia, Shenyang, Liaoning, Zhejiang Lishui, and Qingdao, Shandong. The notice is clear that the funds are used to deepen the comprehensive reform of public hospitals and support high -quality development of public hospitals. According to the "Guiding Opinions on Comprehensive Reform of Urban Public Hospitals" issued by the General Office of the State Council, the comprehensive reform of public hospitals also includes the implementation of the basic construction and equipment purchase of public hospitals, and equipped with corresponding large -scale medical equipment. In addition, the document emphasizes that public hospitals should prioritize domestic medical equipment. The two batches of public hospitals will be facilitated to enter the hospital after the comprehensive reform of the fiscal funds of the comprehensive reform of the public hospital. (Source: Ministry of Finance, the Home of Equipment) 2. The Fujian Provincial Drug Administration issued the "Notice on Promoting the Construction of the Informatization Save System of Chinese Medicine Drinking Pieces"
On June 14, the Fujian Provincial Pharmaceutical Bureau issued a notice on promoting the construction of the information traceability system of traditional Chinese medicine decoction tablets, informing the production enterprise to complete the construction of the traceability system by the end of August 2022, and implement the code traceability of the Chinese medicine decoction tablets produced. By the end of 2022, the construction of the information traceability system for the production management of traditional Chinese medicine beverage films in the province can also be encouraged to use the traceability system provided by third -party technology institutions to trace traceability for the production of Chinese medicine drinking tablets. Effectively improve the quality and safety guarantee level of Chinese medicine drinks. (Source: Fujian Provincial Drug Administration)
(2) Industry News
1. Haikou is bigger and stronger medical device industry. Five companies have signed contracts to settle in high -tech zones
On June 18, 2022 Hainan Free Trade and Hong Kong Medical Device Industry Development and Docking Meeting was held in Haikou. Five companies signed on -site to settle in the medical device park in Haikou Haikou High -tech Zone. This docking conference concentrated contract project is the third -party testing platform project of the medical device of SGS Tongzhong Standard Technology Service Co., Ltd., Derong Medical Technology Co., Ltd. Derong Digital Smart Warehouse Equipment Center, Hunan Xiangyi Lab Instrument Development The high -end medical centrifuge production base of the limited company and the orthopedic consumables research and development and production base project of the Chinese Medicine Equipment Panjin Co., Ltd., and the anti -serum product R & D and production base project of Shenzhen Qianhai Tianzheng Biotechnology Co., Ltd. The total industrial output value of biomedical enterprises in Haikou City accounts for 95%of the province's total output value. From project recruitment, corporate cultivation, and research and development capabilities, to the continuous optimization of the industrial environment, Haikou deepen the reform of "decentralized service". (Source: Science and Technology Daily)
2. Heilongjiang Provincial Pharmaceutical Supervision Bureau released the ability comparison plan of laboratory in 2022
On June 14, the Heilongjiang Provincial Pharmaceutical Bureau issued a notice of capability comparison plan inter -laboratory capacity comparison plan in 2022, requiring the province to have a inspection and testing institution with comparison project detection qualifications and the quality control laboratory of production and development enterprises in the corresponding areas. Improve the ability of drugs, medical devices, cosmetics inspection and testing capabilities under the new situation in the new situation of the local city inspection and testing agency, establish a high -level inspection technology support system, and enhance the quality control awareness of the quality control laboratory of production and research and development enterprises. (Source: Heilongjiang Provincial Drug Administration)
3. The State Drug Administration convened a medical device network transaction management risk meeting
On June 14, the State Drug Administration held a chamber of commerce by the medical device network transaction management risk. The meeting pointed out that the third -party platform of medical device network transaction services should strictly implement the main responsibility of the platform, ensure the quality and safety of the online sales of medical devices, strengthen internal management and personnel training, and ensure the continuous compliance of online transaction services. Strict qualification review should be strictly entered. Carefully checking the business license, registration certificate, and filing voucher information of the enterprise medical device business must be comprehensive, accurate, complete, and meticulous. If necessary, consult the issuance department and verify it. "Outside the door." To strengthen the frequency and intensity of the monitoring information of the network transaction information in the platform, and discover the violation of the "Regulations on the Supervision and Administration of Medical Device" and "Measures for the Supervision and Administration of Medical Device Network Sales Supervision and Management", measures should be taken immediately Suspected illegal and violations (Source: State Drug Administration)
(3) Overseas information
1. Electro -physiological guidance company Agile MV was acquired by Corey
On June 14th, the RESONETICS company supported by GTCR announced with Carlyle had acquired the Agile MV, and the specific acquisition amount was not disclosed. Agile MV is headquartered in Canada. It was established in 2010. It is dedicated to the design, development and manufacturing of minimally invasive diagnosis and therapeutic medical equipment. It is committed to providing product development and equipment assembly in electrical science and intervention of cardiac disease catheter markets. (Source: Siyu MedTech)
2. RESMED announced the acquisition of German medical software developer Medifox Dan
On June 14, RESMED announced the acquisition of Medifox Dan, a German medical software developer, with a transaction amount of US $ 1 billion. Established in 1989, Ruisimai is a professional manufacturing company of sleep respiratory equipment. The cloud connection medical equipment developed by it has changed the nursing method of sleep respiratory suspension, COPD and other patients with chronic diseases. Nursing staff provides support. Medifox Dan is a company that develops software for outside nursing institutions. Products include nursing plans, personnel plans, administrative management, etc. The catalyst of this transaction is the large -scale continuous recall of Philips' sleep ventilator. In order to fill the gap in the market, the huge demand of Ruisimai Sleep ventilator has promoted the generation of the merger. Completed before. Source: Siyu MedTech) 3. The latest SPECT/CT system for Siemens is approved by FDA
On June 13, the latest Spect/CT system of Siemens Healthiners was approved by FDA. The system combined SPECT and CT imaging into a relatively small device, Symbia Pro.Specta. The core of Symbia Pro.Specta is MyExam Companion, which includes an intuitive user interface that eliminates traditional manual and dependent on users' SPECT/CT imaging workflows. MyExam Companion provides automation tools to guide users to complete each step of inspection and decision -making process. From system and patients to image collection and reconstruction, to evaluation and post -processing. Therefore, no matter what the patient, program, or user experience level, the department can get consistent results quickly and easier. Symbia Pro.Specta aims to replace Symbia Intevo series SPECT/CT machines of Siemens. This series of machines was first approved for FDA for the first time in 2013. (Source: Siyu MedTech)
appendix:
Appendix 1: Digital medical enterprise stock price fluctuation increase list
(2022/6/10-2022/6/17)
Data source: Zero One Think Tank
Appendix 2: Digital medical enterprise stock price fluctuation drop list

(2022/6/10-2022/6/17)
Data source: Zero One Think Tank
Statistical explanation
1. The digital medical company defined in this report refers to innovative enterprises that apply modern computer technology, information technology to medical, biomedical and medical equipment. , Mobile Internet and other technologies provide digital products, services or technical solutions for medical institutions, biomedical and medical device production and development enterprises, covering medical institutions' information services, Internet medical (smart medical), digital health management, pharmaceutical e -commerce companies , Medical Robot, AI Pharmaceutical, AI Medical Devices and other aspects.
2. The equity financing referred to in this report includes the private equity financing before Pre-IPO of the equity crowdfunding, seed/angel, Pre-A, A, A+to Pre-IPO, excluding convertible bonds, mergers and acquisitions, new three boards, and new three boards fixed increases , IPO, IPO+, etc.
3、本报告对于未披露具体金额的融资处理方式为:未透露=0,数十万=50万,数/近百万=100万,数/近千万=1000万,数/近亿/ 100 million yuan and above = 100 million, billions = 1 billion.
4. In order to facilitate statistics, when currency conversion, this report ignores short -term changes in the exchange rate. The conversion method between different currencies is: 1 dollar = 7 yuan, 1 euro = 8 yuan, 1 pound = 8.8 yuan, 1 rupee = 0.09 Yuan, 1 won = 0.006 yuan, 1 plus dollar = 5 yuan, 1 Swiss franc = 7 yuan, HK $ 1 = 0.8 yuan, 1 Swedish Crane = 0.8 yuan, 1 yen = 0.06 yuan, 1 Australian dollar = 4.86 yuan.
5. All the data in this report comes from the public channel disclosure. Due to possible omissions or delay, we will trace back to the previous statistics, and no longer update the release of the published reports.
End.
- END -
latest news

The latest notice of the National Health and Health Commission! Yesterday, there w...
Crossing 1,000 kilometers of heart protection journey!22 -year -old college student "changed his heart" to be reborn

The Yangtze River Daily Da Wuhan Client June 22 (Correspondent Ning Yafei reporter...