2022 WCLC | Wonderful hotspots -Flaura and Aura series studies long -term drug tolerance and safety analysis
Author:Cancer Channel of the Medical Time:2022.09.04
*For medical professionals for reading reference
The latest research retrospective analyzes the tolerance and safety of long -term oral oral oxininib.
The highly anticipated annual World Lung -Cancer Conference (WCLC) was held in Vienna, Austria from August 6th to 9th. The conference was hosted by the International Lonopolic Cancer Research Society (IASLC) and gathered the most authoritative experts in the global lung cancer field. Scholars and front -line clinical doctors. At this conference, the third representative of the third representative of the epidermal growth factor receptor (EGFR) -atheosine kinase inhibitor (TKI) Oshitinib in the advanced non-small cell lung carcinoma (NSCLC) of EGFR mutations (EGFRM) Still discussing hotspots.
Aura series studies [1-3] and Flaura studies [4] Data show that patients with Oshitinib the treatment of EGFRM NSCLC can bring long-term benefits, but the security of patients with Oshitinib for a long time has not yet been analyzed by the security of patients with Oshitinib for a long time. Essence In a study published by 2022 WCLC [5], based on the starting point, retrospective analyzes the occurrence of Aura, Aura2, Aura3, and Flaura research. Essence
Research methods
Studies have included patients from Aura series (including Aura Studies, Aura2 Studies, AURA3 Studies in patients with Oshitinib therapy, and patients who use Oshitinib for first -tier treatment in Flaura. All patients receive Oshis Tetinib 80mg QD treatment is allowed to reduce or interrupt medications due to adverse events (AES).
Researchers conducted relevant data analysis of patients who used Olkinib for more than 36 months. After the final data of the above clinical trials, the global drug safety database is still tracking patients who continue to use Oshitinib to record the serious bad events (SAE) reported by the researcher. However, Chinese patients are excluded from this analysis due to export restrictions.
The retrospective studies selected the best relief data from the 54 -month and 36 -month patients from the Flaura research and Aura series of research, respectively. Flaura's median PFS for the treatment of patients in Oshitinib was 18.9 months [4], and Aura3 studied the median PFS for 10.1 months [3]. The study of Oshitininib was at least 3 times the medium PFS patient data of these two studies to reflect these groups of people who may benefit for a long time. Data from Flaura Research and Aura3 Studies are evaluated by researchers, while Aura Studies and AURA2 Studies are reviewed and evaluated by Blind Independent Center. Research schematic refer to Figure 1.
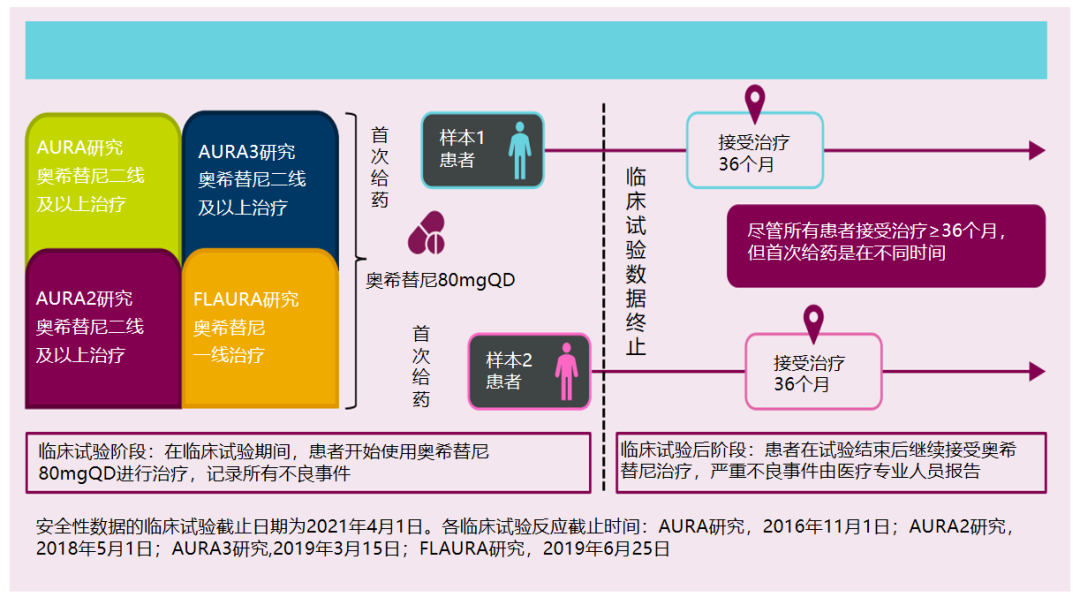
figure 1
Research result
In the case of patients' population statistics and baseline diseases and their own overall trials, Flaura studied the Communist Party of China into 267 patients, of which 76 patients (28%) received Oshitininib for ≥36 months, 36 cases of 36 cases, 36 cases Patients received Oshitinib for ≥54 months. Among them, the median exposure duration of patients with ≥36 and ≥54 groups was 52.5 (range: 37.4-70.2) a month and 64.5 (54.5-70.2) months, respectively.
AURA series studies were included in 799 patients, of which 124 patients (16%) were treated with Oshitinib for ≥36 months (43 AURA studies, 35 AURA2 studies, and 46 AURA3 studies). The exposure duration is 44.7 (36.0-81.2) a month.
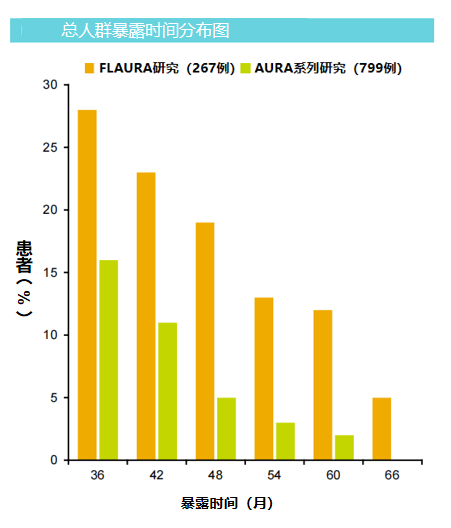
figure 2
Security analysis
In the clinical trial stage, all SAEs were reported in patients with oxininib therapy ≥ 36 months. There were 13 cases (17%) in Flaura studies, and 44 cases (35%) in the Aura series of studies, of which SAE related to treatment was 2 (3%) and 6 (5%).
Table 1 Safety data in clinical trial stage
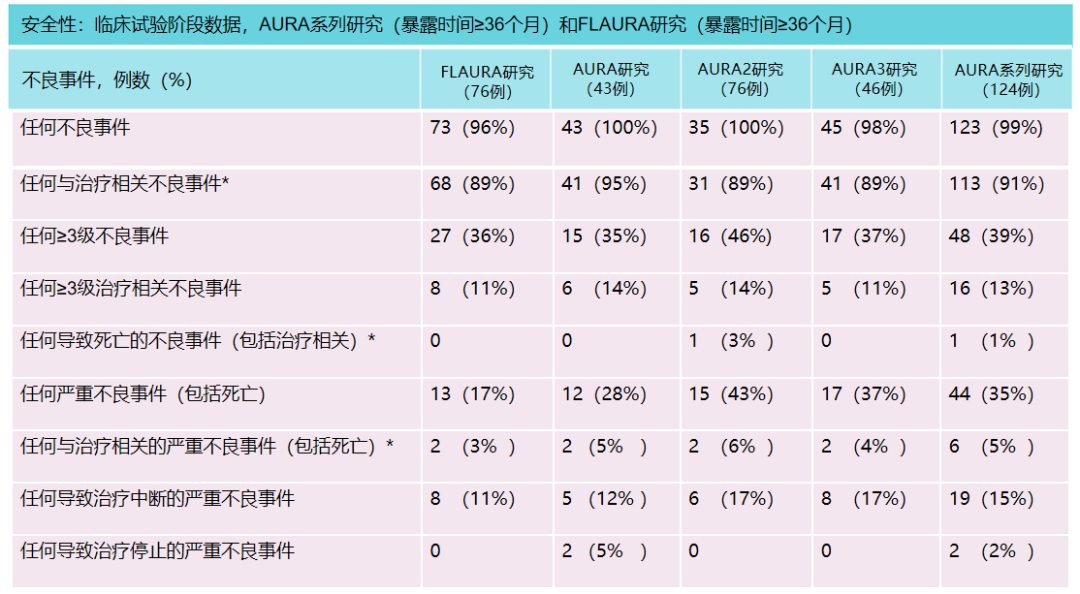
Note 1: Patients may report multiple serious adverse events, with*symbols for evaluation by researchers
After the clinical trial deadline, it is still continuing to receive patients with Oshitinib for ≥36 months. The data extracted from the global database is consistent with clinical trial data. There were 8 cases (17%) in Flaura's research, and 26 cases (21%) in the Aura series studies.
Table 2 Safety data after clinical trial termination
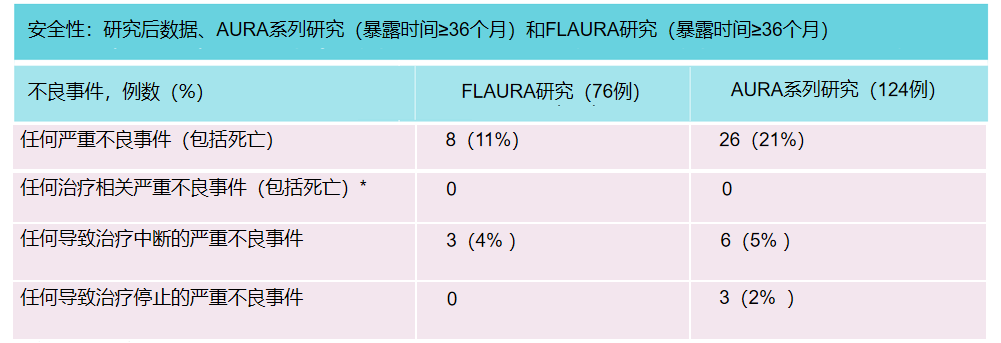
Note 2: Safety data, medication reactions and results after clinical trial termination are not completely recorded. Among them, the actions of two serious adverse events are unknown. Bring*symbols are evaluated by researchers
Efficacy analysis
From the clinical trial data of Flaura research and Aura series of research, most of the patients who have long been treated with Oshitinib for a long time have reached partial relief (PR). Patients who received ≥54 months in the Flaura study received ≥54 months, and 86%of patients received PR; patients who were treated with Oshitinib for ≥36 months in AURA series studies, Aura2 and Aura3 research obtained PR patients accounted for 80%and 83%, respectively.
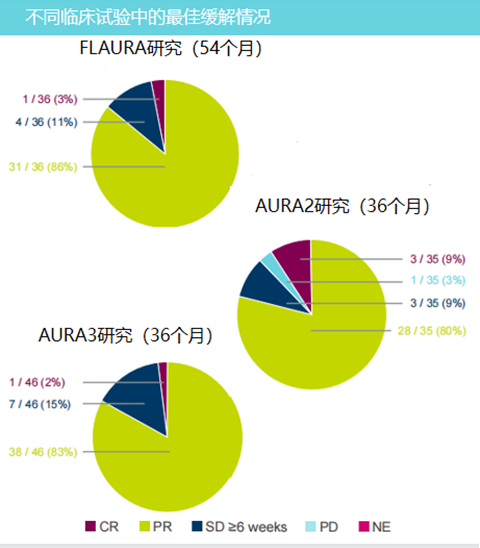
image 3
Summarize
The study retrospectively analyzed the data of Flaura research and Aura series of research, and found that in Osteini's long -term treatment, its safety and efficacy have obtained a pleasing result. In terms of security, there are only 6 cases related to treatment related to the treatment of AURA series of AURA series of AURA series. There are only 6 cases related to treatment related to treatment. There are only 2 cases in Flaura studies, and their incidence is only 5%and 3%, respectively. Essence In terms of efficacy, 86%of patients received PR in Flaura's research receiving Oshitinib ≥54 months; patients who received ≥36 months in Aura series studies in Aura series, Aura2 and Aura3 studies obtained PR Patients account for 80%and 83%. Although the data obtained in the global security database may lack integrity after clinical trial termination, the overall results show that Osicinib usually tolerates good tolerance and safety among patients who receive long -term treatment for 3 years or more. This material is supported by Astrikon, for medical professionals for reference
Approval number: CN-101644 Expired Date: 2022-12-1
references:
[1] .Yang JC, Ahn MJ, Kim dw, et al. Osimertinib in pretreated T790M-POSITIVE Advanced Non-Small-CLL LUNG CANCER: Aura Study Phase II Extension Component. J Clin oncol. : 1288-1296.
[2] .goss g, tsai cm, shepherd fa, et al. Osimertinib for pretreated egfr Thr790Met-POSITIVANCED NON-Small LUNG CANGER (AURA2): A Multiplentre, Open-Label, SINGLEM. Lancet oncol. 2016 DEC; 17 (12): 1643-1652.
[3] .mok TS, wu y-l, ahn m-j, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-POSITIVE LUNG CANCER. N ENGL J Med. 2017 Feb 16; 376 (7): 629-640.
[4] .soria JC, OHE Y, VANSTEENKISTE J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell LUNG CANCER. N English. 2018 Jan 11; 378 (2). 113-125.
[5].Marina Chiara Garassino , Yong He , Myung-Ju Ahn,et al. Osimertinib long-term tolerability in patients with EGFRm NSCLC enrolled in the AURA program or FLAURA study.2022WCLC.EP08.02-108.
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
Is there a problem with the kidney in the urine?Listen to the doctor what to say

I don't know if everyone goes to the toilet (regardless of the size), is it used t...
Starting at 00:00 on August 4, Sanya, Hainan entered the regional control status
Sanya new coronary virus pneumonia epidemic prevention and control work headquarters notice(Notice No. 54, 2022)Citizen tourists:The current situation of the epidemic in Sanya is severe and complicate