Precisely block the early intervention of Azf, which is determined to provide powerful weapons for the prevention and control of the new crown epidemic
Author:Medical newspaper Time:2022.09.04
Physician newspaper (Rong Media Reporter Qiu Jia) The new crown epidemic has a huge impact on people's health and production and life. As of August 2022, more than 580 million patients in the world were infected with more than 6.4 million deaths; The number of excess deaths caused by the outbreak of the new crown epidemic in the month was about 14.9 million. The continuous mutation of the virus has caused layers of obstacles to the complete end of the epidemic.
According to the popular experience of influenza, the new crown oral antiviral drugs are an important part of solving the prevention and control of the epidemic. A few days ago, my country ’s first 1.1 type 1.1 category 1.1 treatment of new crown pneumonia small molecules oral drugs with a global patent Azf will be born, filling the gap in my country's pharmaceutical industry in the field of new coronary virus pneumonia. Control the epidemic and provide strong support for people's health. The ninth edition of the "New Coronatte Pneumonic Pneumonia Diagnosis and Treatment Plan" emphasizes the concept of "movement forward", and the use of antiviral small molecular drugs is added to the treatment plan. On August 9, the National Health and Health Commission issued a notice that Azf's fixed tablet was included in the new type of coronary virus pneumonia diagnosis and treatment plan for the treatment of patients with ordinary adult new coronary pneumonia.
How is Azf's Dingxin New Crown Pneumonic Pneumonic Molecular oral drugs antivirus? What is its efficacy and safety? On September 3rd, at the 14th Chinese Physician Association Infectious Medicine Conference and the ability to improve the ability of infected subjects, Fosun Pharmaceutical Special Special Conference, Azf's new crown indication development led PI, Capital Medical University affiliated to the Capital Medical University University Professor Zhang Fujie, Beijing Diquan Hospital, brought a detailed introduction on the mechanism of Azf's new crown virus and the results of clinical research at home and abroad. Professor Wang Guiqiang, the First Hospital of Peking University, served as the host of this link.


The two ways of "help right" and dispel evil "
Lay the foundation for the efficacy
"Previous studies have shown that patients usually experience symptoms in the 5-6 days (incubation period) after the first new coronary virus is exposed. In the respiratory tract, the virus load value is observed during the symptoms or on the first week of the disease, and then decreased. Before or before the symptoms have the highest contagious potential. "
Professor Zhang introduced that in the treatment of new crown pneumonia, three types of drugs have received much attention: small molecular drugs, nucleoside drugs, and antibody drugs. Among them, oral antiviral small molecular drugs are convenient for administration and moderate prices, which are still significant for mutant virus strains. It is an important weapon for the prevention and control of new coronary pneumonia. Azf is a oral antiviral small molecular nucleoside analog.
Azf is designated as a wide -spectrum RNA virus inhibitor, which can inhibit the new coronary virus RNA dependencular RNA polymerase (RDRP) and block the extension of RNA. Because RNA synthesis is blocked, there is no risk of virus or host mutation. "So we use antiviral drugs to be invalid for those who have copied viruses, and can only prevent the virus being replicated. All antiviral drugs work." Professor Zhang said.
In addition, thymus is very important for maintaining normal immune function. Azf will reduce the load of thymic virus and activate CD4 +and CD8 +cells. Release, thereby reducing the inflammatory response and organ damage.
Three global clinical trials confirmed
Azf will have good efficacy and safety
In the early days of outbreak of the epidemic in 2020, Professor Peng Xiaozhong, Kunming Experimental Center of the Institute of Medical Biology of the Chinese Academy of Medical Sciences, and Academician Jiang Jiandong jointly conducted in vitro experiments, proved that Azfdin contracted Rydecow, which was equal to the inhibitory effect of the new coronary virus. Subsequently, the stage I test observed a series of dynamics, tolerance, and pharmacokinetics of the administration, as well as many studies such as administration, pre -meal after meals, and other studies. Safe tolerance.
"As of now, Azf has a global clinical trial that has launched anti -new coronal viruses in China, Russia, and Brazil." Professor Zhang introduced that my country ’s research was led by the Beijing Earth Altar Hospital affiliated to the Capital Medical University and participated in 11 centers across the country. Multi -centered, random, double -blind, and placebo -controlled research is the highest standard clinical trial of new drugs. Study selected for light and ordinary patients from 18 to 75 years old, 5 mg/d in Azfdin/placebo, and treatment of 7 ~ 14 d. The main efficacy indicators are virus loads. A total of 327 patients were included, and no death occurred during the treatment.
The results of the research show that the test group subjects are analyzed in the sub -group analysis according to the baseline virus load LOG value> 2, 3, and 4. The higher the load of the baseline virus, the stronger the inhibitory virus effect. On the fifth day of the virus, the decrease in the load of the virus was significantly higher than that of the control group.

The central data of the Di Tan Hospital is the same. The virus load decrease value is greater than the control group on the 3rd, 5th and 7th days, and the 5th day is statistically significant. A subject with a baseline virus load LOG ≥ 3, the decreased difference in the virus load on the fifth day was 17.7 times that of the control group.
In terms of security, compared with the control group, there are no adverse reactions and deaths above three levels. The two groups have no significant differences in statistics. Explain that Azfdin's tolerance and safety are good.
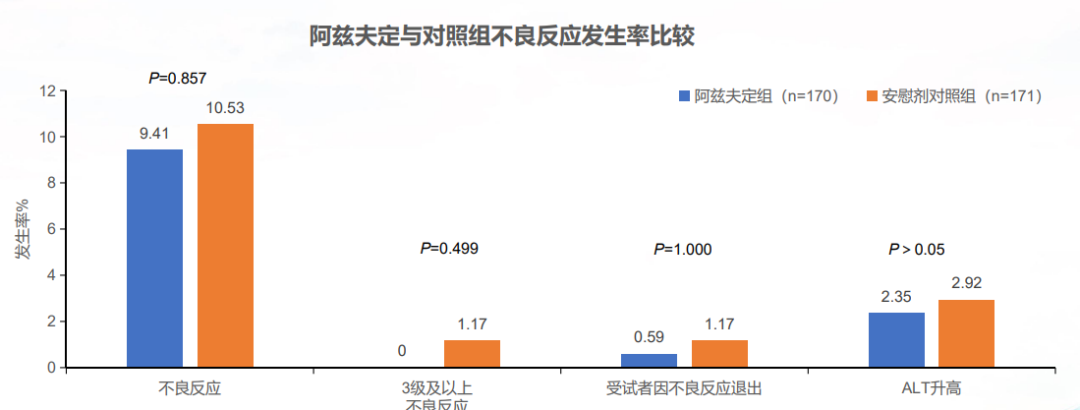
The research conducted by Brazil is to evaluate the efficacy and safety of Azf's moderate to moderate to severe infection patients. Mainly observe the improvement of clinical symptoms on the 15th day. The study was incorporated into 180 subjects. The results showed that the test group's final clinical improvement was significantly higher than the subjects of the control group, and the nucleic acid rotation time and hospitalization time were significantly lower than the control group subjects. Russia's research was included in 314 patients with medium -sized infection to observe the proportion and time of the symptoms on the seventh day. The results showed that the proportion of the clinical condition of the test group was much higher than that of the control group (40.43%and 10.87%), and the clinical improvement time was significantly lower than the control group (10 d and 13 d). In terms of security, there is no significant difference in the two groups.
In summary, there are three clinical trial results. It can be seen that Azf will have the effect of early inhibitory virus, which significantly reduces the virus load; it can improve clinical symptoms, shorten the hospitalization time, reduce the time of turning the yin, and good safety. The help of recovery should leave the cabin as soon as possible and conduct home health observations at the community.
Professor Zhang said that after the infection of the new crown virus, the virus is effectively controlled, which can reduce the risk of transmission, and effectively control the development of the disease. In addition to reducing the risk of intensiveness and critical illness through antiviral therapy, and reducing symptoms, the drug will also do further research in patients such as children, liver and kidney failure, etc. in the future. At the same time, it is also worth further exploration after exposure. Looking forward to further digging the clinical application value of drugs and adding bricks to the epidemic prevention and control.
In the summary, Professor Wang Guiqiang said that vaccination and antiviral small molecular drugs are important factor for changes in the prevention and control strategy of the epidemic. Azf has been listed and has been added to the ninth edition of the treatment plan, providing a very good weapon for the treatment of new crown pneumonia. In the future, we hope to continuously improve the role of drugs in the new crown treatment in clinical practice.
Capture: Qiu Jiajia
Edit: Qiu Jiajia
- END -
Another batch of drugs price adjustment!

June 27thWuhan Medical Insurance Bureau issued noticeAccording to the unified requ...
Guiyang screened 301 positives, and there was a spillover of the spread chain!An emergency announcement in Henan: These people are home in principle ...

Guiyang screened 301 positive! The Huaguo epidemic has spilled and has been enable...