The new regulations for drug network sales will implement the real -name system
Author:Dahe Cai Cube Time:2022.09.01
[Dahecai Cube News] Recently, the General Administration of Market Supervision issued the "Measures for the Supervision and Administration of Drug Network Sales" (Order 58 of the State Administration of Market Supervision and Administration), which will be implemented from December 1, 2022.
Drug safety responsibility is greatly responsible for the lives and health of the people, and the Party Central Committee and the State Council attach great importance to it. In order to implement the "four most stringent" requirements of the Party Central Committee and the State Council and a series of decision -making deployments, refine the regulations on drug network sales, and coordinate the convenience of drug purchases and drug safety supervision, and effectively ensure the protection of the public. The public drug safety and legitimate rights and interests, the General Administration of Market Supervision and the Drug Administration formulated the "Measures for the Supervision and Administration of Drug Network Sales Sales" on the basis of in -depth research and fully demonstration. "Measures" total 6 chapters and 42 articles, and stipulates the management of drug network sales, platform responsibilities, supervision and inspection measures and legal responsibilities. The main contents include:
The first is to implement the main responsibility of drug management enterprises. Clarify the qualifications and requirements of the pharmaceutical business enterprise engaged in drug network sales, and clarify the vaccine, blood products, anesthesia drugs, psychotropic drugs, medical drugs, radioactive drugs, pharmaceutical and chemicals and other countries in accordance with the law Do not sell on the Internet. At the same time, strict management of drug operations, and put forward clear requirements for the management system of drug network sales enterprises, pharmaceutical services, pharmaceutical storage and distribution, pharmaceutical traceability, risk control, and information disclosure of drug network sales enterprises.
The second is the responsibility of compacting the drug network sales platform. Clarify that third -party platforms should set up drug quality and safety management institutions, equipped with pharmacy and technical personnel, and establish and implement drug quality and safety, pharmaceutical information display, prescription review, prescription drug real -name purchase, drug distribution, transaction record preservation, adverse reaction report, complaint report processing, etc. Management system and record in accordance with regulations. At the same time, the platform is required to sign an agreement with the drug network sales enterprise to clarify the responsibility of drug quality and safety between the two parties. It stipulates that the platform shall perform the obligations and reports of the stop service and report of the suspension of the audit, inspection and monitoring, and the discovery of serious violations. Emergency treatment and coordination obligations in supervision and inspection.
The third is to clarify the management of prescription drug network sales. Considering the safety risks of medication and online consistency management requirements, clearly implement a real -name system for the sales of prescription drug networks, and conduct prescription review and deployment in accordance with regulations. Display packaging, labels and other information; before the prescription review, no information such as instructions shall be displayed, and the relevant services of prescription medicine purchase shall not be provided. It is intended to emphasize the management requirements of "first medicine" and prescription review. At the same time, before the sales of prescription drugs, consumers should fully inform consumers to fully inform the relevant risk warning information and confirm their knowledge by consumers, and effectively prevent the safety risk of medication.
The fourth is to implement the "four strictests" requirements and strengthen supervision measures of regulatory departments at all levels. Clarify the duties of drug supervision and management departments at all levels in the division of responsibilities in drug network sales and the jurisdiction of illegal acts. It is required to strengthen the monitoring of drug network sales, and investigate and dispose of illegal acts found in accordance with the law in accordance with the law. To strengthen the control of drug safety risks, if there is a hidden safety hazard in the evidence, it is clear that the drug regulatory authorities can adopt advice, interviews, rectification within a time limit, and suspending production, sales, use, and imports. In addition, the "Measures" also clarified the corresponding legal liability for the illegal act of drug network sales.
Attachment:
Measures for the supervision and management of pharmaceutical network sales
(August 3, 2022 announced on the 58th of the State Administration of Market Supervision and Management
From December 1, 2022)
Chapter 1 General Principles
Article 1 In order to regulate the service activities of drug network sales and drug network trading platforms and ensure the safety of public medication, these measures are formulated in accordance with laws and administrative regulations such as the Drug Management Law of the People's Republic of China (hereinafter referred to as the Drug Management Law).
Article 2 It shall comply with these Measures to engage in drug network sales, provide drug network trading platform services and their supervision and management in the territory of the People's Republic of China.
Article 3 The supervision and management of national pharmaceutical network sales in charge of the national pharmaceutical supervision and administration bureau.
The provincial drug supervision and management department is responsible for the supervision and management of drug network sales in the administrative region, and is responsible for supervising and managing the third -party platform of the drug network transaction and the activities of the drug listing license holder and the drug wholesale enterprise to sell drugs through the network.
The municipal and county -level municipal and county -level departments (hereinafter referred to as drug supervision and management departments) at the municipal and county levels are responsible for the supervision and management of drug network sales in the administrative region, and the activities of supervising and management of retail enterprises to sell drugs through the network.
Article 4 Cainting drug network sales and providing pharmaceutical network trading platform services shall comply with pharmaceutical laws, regulations, rules, standards, standards and specifications, and operate in accordance with the law to ensure the quality and safety of drugs.
Article 5 The services engaged in drug network sales and providing pharmaceutical network trading platform shall take effective measures to ensure that the information of the entire process of transaction is true, accurate, complete, and traceable, and abide by the relevant regulations of national personal information protection.
Article 6 The drug supervision and management department shall strengthen collaboration with relevant departments, give full play to the role of industry organizations and other institutions, promote the construction of a credit system, and promote social co -governance.
Chapter 2 Drug Network Sales Management
Article 7 If you are engaged in the sales of drug networks, it shall be a drug listing license holder or a drug business enterprise with a drug -saving drug safety capacity. Traditional Chinese medicine production enterprises selling Chinese medicine drinking tablets should fulfill the obligations of the holder of the drug listing permit.
Article 8 Drug network sales enterprises shall operate in accordance with the approval operating method and business scope. Pharmaceutical network sales enterprises who are a drug listing license holder can only sell drugs that obtain drug registration certificates. Those who have not obtained drug retail qualifications shall not sell drugs to individuals.
Pharmaceuticals such as vaccine, blood products, anesthesia drugs, psychotropic drugs, medical drugs, radioactive drugs, and pharmaceuticals and vulnerable chemicals shall not be sold on the Internet. The specific directory shall be formulated by the State Drug Administration.
Pharmaceutical network retail enterprises shall not violate regulations to give individuals and category A non -prescription drugs to individuals by buying drugs and giving medicines.
Article 9 Anyone who sells prescriptions to individual sales through the Internet shall ensure that the source of the prescription is true and reliable, and a real -name system shall be implemented.
Pharmaceutical network retail enterprises shall sign an agreement with the provision of the unit with electronic prescriptions, and strictly conduct prescription review and deployment in accordance with relevant regulations, and mark the electronic prescriptions that have been used to avoid reuse of prescriptions.
If a third -party platform undertakes electronic prescriptions, the situation of the unit provided by the electronics prescription shall verify and sign an agreement.
If the prescription received by a pharmaceutical network retail enterprise is a papermaking version of the paper prescription, effective measures should be taken to avoid reuse of prescriptions.
Article 10 Drug network sales enterprises shall establish and implement systems such as drug quality and safety management, risk control, pharmaceutical traceability, storage and distribution management, adverse reaction reports, and complaint report processing and other systems.
Pharmaceutical network retail enterprises should also establish an online pharmaceutical service system. Pharmacologists or other pharmacy and technicians who have been qualified by law to carry out prescription review and deployment, and guide rational medication. The number of pharmacists or other pharmacy or other pharmacy and technicians in accordance with the law shall be compatible with the scale of business.
Article 11 Drug network sales enterprises shall report information such as corporate names, website names, application names, IP addresses, domain names, pharmaceutical production licenses, or pharmaceutical business licenses to the drug supervision and management department. If the information changes, it should be reported within 10 working days.
Pharmaceutical network sales enterprises shall report to the provincial drug supervision and management department of the local provincial drug supervision and management department if they are a pharmaceutical listing license holder or drug wholesale enterprise. If a pharmaceutical network sales enterprise is a pharmaceutical retail company, it shall report to the county -level drug supervision and management department of the city.
Article 12 Pharmaceutical network sales enterprises shall continue to publicize their pharmaceutical production or business license information on the homepage of the homepage or business activities. Pharmaceutical network retail enterprises should also show information such as the qualification identification of pharmacists or other pharmacy and technical personnel equipped in accordance with the law. If the above information changes, it should be updated within 10 working days.
Article 13 Drug -related information displayed by pharmaceutical network sales enterprises shall be true, accurate and legal.
Pharmaceutical network retail companies engaged in prescription drugs should highlight risk warning information such as "prescription drugs must be purchased and used under the guidance of pharmacists under the guidance of pharmacists" under each drug display page. Before the preparation of prescription drugs, it shall fully inform consumers to tell the relevant risk warning information and confirm the knowledge by consumers.
Pharmaceutical network retail enterprises should distinguish prescription drugs from non -prescription drugs, and significantly indicate prescription drugs and non -prescription drugs on the related webpages.
Pharmaceutical network retail companies shall not directly display information such as prescription drug packaging, labels and other information on the homepage of prescription drug sales. Before the prescription review, no information such as the manual shall be displayed, and the relevant services of the prescription medicine purchase shall not be provided.
Article 14 Drug network retail enterprises shall be responsible for the quality and safety of drug distribution. Delivery drugs should choose appropriate transportation tools and facilities according to the number of medicines, transportation distance, transportation time, temperature and humidity requirements, etc., and the delivery drugs should be placed in an independent space and obviously identified to ensure that the requirements meet the requirements and trace the whole process.
If the pharmaceutical network retail enterprise entrusted distribution, the quality management system of the trustee shall be reviewed, and the quality agreement with the trustee shall be signed with the trustee's quality responsibility, operating procedures, etc., and supervise the recipient.
The specific distribution requirements of drug network retail are formulated separately by the State Drug Administration.
Article 15 If you sell drugs to individuals, the sales voucher shall be issued in accordance with regulations. Sales vouchers can be issued in the form of electronic. The sales record of the minimum sales unit of the drug should be clearly retained to ensure traceability.
Pharmaceutical network sales enterprises should fully preserve records of supplier qualification documents and electronic transactions. Pharmaceutical network retail enterprises for sales prescriptions should also save records and online pharmacy services. The relevant record preservation period is not less than 5 years, and no less than one year after the validity period of the drug is expired.
Article 16 Drug network sales enterprises shall take corresponding risk control measures in accordance with the law for drugs with quality problems or safety hazards, and will timely disclose corresponding information on the homepage of the website or the homepage of the business activity.
Chapter 3 Platform Management
Article 17 The third -party platform shall establish a pharmaceutical quality and safety management institution, equipped with pharmacy and technical personnel to undertake drug quality and safety management, establish and implement drug quality and safety, drug information display, prescription review, prescription drug real -name purchase, drug distribution, transaction record preservation , Adverse reaction reports, complaint report handling and other management systems. The third -party platform should strengthen the inspection, and manage the drug information display, prescription review, drug sales and distribution of pharmaceutical network sales enterprises that settled in the platform, and urge them to strictly fulfill their legal obligations.
Article 18 The third -party platform shall record the company name, legal representative, unified social credit code, website name, and domain names to the provincial drug supervision and management department where the platform is located. Provincial drug supervision and management departments shall publicize the platform filing information.
Article 19 The third -party platform shall be prominent on the homepage of its website or the homepage of drug business activities, and continue to publicize the information of business licenses, relevant administrative licenses and filing, contact information, complaint reporting methods, or the above information.
The display of the drug information of the third -party platform shall comply with the provisions of Article 13 of these Measures.
Article 20 The third -party platform shall review the qualifications, quality and safety assurance capabilities of the pharmaceutical network sales enterprise applied to settle in, and establish a registration file for pharmaceutical network sales enterprises. At least six months of verification and update Enterprises meet legal requirements.
The third -party platform shall sign an agreement with the pharmaceutical network sales enterprise to clarify the quality and safety responsibilities of the pharmaceutical quality of both parties.
Article 21 The third -party platform shall preserve information such as drug display, transaction records, and complaint reports. The storage period is not less than 5 years, and no less than one year after the effective period of the drug is expired. The third -party platform should ensure the authenticity and integrity of relevant information, information and data, and provide facilitation for the data network sales enterprises where they settled in.
Article 22 The third -party platform shall establish an inspection and monitoring system for drug network sales activities. If there are illegal acts in the drug network sales enterprise settled in, it should be stopped in time and immediately reports to the county -level drug supervision and management department in the local area.
Article 23 If a third -party platform finds the following serious illegal acts, it shall immediately stop providing online trading platform services and stop displaying the relevant information of drugs:
(1) Those who do not have qualifications to sell drugs;
(2) In violation of the provisions of Article 8 of these Measures, those who sell special management of countries shall be implemented;
(3) Sales of drugs exceeding the scope of drug operations;
(4) If the drug supervision and management department is ordered to stop the sales, revoke the approval document of the drug or revoke the drug business license due to illegal acts;
(5) Other serious violations.
If the pharmaceutical registration certificate is revoked and canceled in accordance with the law, the information of the relevant drugs shall not be displayed.
Article 24 When emergencies of public health incidents or other serious threats to public health, third -party platforms and pharmaceutical network sales enterprises shall comply with relevant state emergency response regulations, and take corresponding control and disposal measures in accordance with the law.
If the holder of the drug listing license recalls the medicine in accordance with the law, the third -party platform and the drug network sales enterprise shall actively cooperate.
Article 25 When the drug supervision and management department carries out supervision and inspection, case investigation, incident disposal, etc., a third -party platform shall cooperate. If the pharmaceutical supervision and management department finds that there is an illegal act of a pharmaceutical network sales enterprise, if the third -party platform is required to take measures to stop the third -party platform in accordance with the law, the third -party platform shall perform relevant obligations in a timely manner.
If the drug supervision and management department provides information such as sellers, sales records, pharmacy services, and traceability in accordance with the requirements of laws and administrative regulations, the third -party platform shall be provided in a timely manner.
Encourage third -party platforms and drug supervision and management departments to establish an automated information reporting mechanism such as open data interfaces.
Chapter 4 Supervision and Inspection
Article 26 The drug supervision and management department shall implement supervision and inspection of third -party platforms and pharmaceutical network sales enterprises in accordance with laws, regulations, and rules in accordance with the provisions of laws, regulations, and rules.
Article 27 When the drug supervision and management department checks the third -party platform and the drug network sales enterprise, the following measures may be taken according to law:
(1) Implement on -site inspection of the relevant places of drug network sales and network platform services;
(2) Sample inspection of drugs sold online;
(3) Ask the relevant personnel to understand the relevant situation of the drug network sales activities;
(4) Check and copy transaction data, contracts, bills, books and other related information in accordance with the law;
(5) For medicines that have evidence to prove that they may endanger human health and their related materials, seizure and seizure measures in accordance with the law;
(6) Other measures that can be taken by laws and regulations.
When necessary, the drug supervision and management department can extend the inspection of units and individuals providing products or services for drug development, production, operation, and use.
Article 28 The investigation and punishment of third -party platforms, drug listing licenses, and drug wholesale enterprises shall be responsible for the investigation and punishment of the illegal behavior of drugs to sell drugs through the Internet. The investigation and punishment of the illegal behavior of drug network retail enterprises shall be responsible for the city and county -level drug supervision and management department.
Drug network sales illegal acts are responsible for investigation and punishment. Those who have caused drug safety incidents or evidence that they may endanger human health due to drug network sales activities. Article 29 The drug supervision and management department shall strengthen the monitoring of drug network sales. The drug network sales monitoring platform established by the provincial pharmaceutical supervision and management department shall achieve data docking with the national pharmaceutical network sales monitoring platform.
The drug supervision and management department shall investigate and dispose of the illegal acts found in accordance with the law.
The technical monitoring records of the drug supervision and management department on online sales illegal acts can be used as an e -data evidence of administrative penalties or administrative measures in accordance with the law.
Article 30 If there is a safety hazard that there may be a safety hazard in evidence, the drug supervision and management department shall admit, interview, interview, rectification within a time limit for drug network sales enterprises or third -party platforms in accordance with the supervision and inspection situation. Import and other measures, and timely announce the results of the inspection processing.
Article 31 The pharmaceutical supervision and management department shall strictly keep the personal information and business secrets provided by the drug network sales enterprise or third -party platform, and shall not be leaked, sold or illegally provided to others.
Chapter 5 Legal Responsibility
Article 32 If laws and administrative regulations shall be stipulated in the punishment of drug network sales illegal acts, they shall be in accordance with its provisions. If the drug supervision and management department finds that the drug network sales are suspected of crime, the case shall be transferred to the public security organs in a timely manner.
Article 33 If you violate the provisions of Article 8 of these Measures, if you implement special management of drugs through online sales countries, if the laws and administrative regulations have stipulated in accordance with the provisions of laws and administrative regulations. If the laws and administrative regulations are not stipulated, they shall be ordered to make corrections within a time limit, and a fine of 50,000 yuan and 100,000 yuan will be imposed; if it will cause the consequences of harm, it shall be fined 100,000 yuan and more than 200,000 yuan.
Article 34 The provisions of Article 9, paragraph 1 and 2 of these Measures shall be ordered to make corrections within a time limit, and a fine of less than 30,000 yuan or not; if the circumstances are serious Essence
In violation of the provisions of Article 9 of these Measures, it is ordered to make corrections within a time limit and impose a fine of 50,000 yuan to 100,000 yuan; if it causes the harmful consequences, a fine of 100,000 yuan and more than 200,000 yuan.
In violation of the provisions of Article 9 of these Measures, it is ordered to make corrections within a time limit and impose a fine of 10,000 yuan to 30,000 yuan; if the circumstances are serious, a fine of 30,000 yuan is 30,000 yuan.
Article 35: In violation of the provisions of Article 11 of these Measures, it shall be ordered to make corrections within a time limit; if the overdue is not corrected, a fine of 10,000 yuan is 30,000 yuan shall be imposed; if the circumstances are serious, a fine shall be imposed.
Article 36 The provisions of Article 13 and 19 of these Measures shall be ordered to make corrections within a time limit; if they do not make corrections within the time limit, a fine of 50,000 yuan is 100,000 yuan.
Article 37 If the provisions of Article 14 and 15 of these Measures, if a pharmaceutical network sales enterprise fails to comply with standardized quality management of drug management, punishment shall be punished in accordance with the provisions of Article 126 of the Drug Management Law.
Article 38 The provisions of Article 17 (1) of these Measures shall be ordered to make corrections within a time limit, and shall be imposed by 30,000 yuan and 100,000 yuan; if the harmful consequences will be caused, a fine of 100,000 yuan is 100,000 yuan.
Article 39: In violation of the provisions of Article 18 of these Measures, it is ordered to make corrections within a time limit; if it is not corrected within the time limit, a fine of 50,000 yuan is 100,000 yuan; if it causes the consequences of harm Essence
Article 40: Article 20, 22, and 23 of these Measures, if the third -party platform fails to perform the obligations of qualification review, report, and stop providing online trading platform services, in accordance with the Drug Management Law Punishment of Article 131.
Article 41 The drug supervision and management department and their staff do not fulfill their duties or abuse their powers, neglect their duties, and have their legal responsibilities in accordance with the law.
Chapter 6
Article 42 These Measures will be implemented from December 1, 2022.
Responsible editor: Shi Jian | Audit: Li Zhen | Director: Wan Junwei
- END -
Mistake the onychomycosis as the eye drops, and the two men's eyes were chemically burned
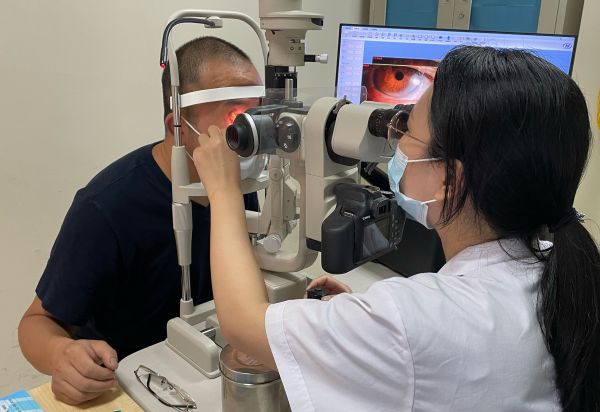
Wuhan Evening News, June 17 (Correspondent Ao Panpan) Mr. Liu of Wuhan treats the ...
up to date!Hubei Disease Control and Prevention Releases Health Management Measures for Hubei Hubei

I. For the people who come to Hubei in the history of domestic and high -risk area...