Inventor interprets Azfding
Author:Dahe Health News Time:2022.08.31
At the press conference of the "China Ten Years of China · Henan" of the Henan Provincial Party Committee of the Communist Party of China, the treatment of new crown pneumonia's small molecules -Azf was approved to be listed as the top ten innovation achievements in Henan.
Why can Azf get so honored? What role will Azf play in the future? Azf's inventor, former president of Henan Normal University, Deputy Secretary of the Party Committee and Vice President of Zhengzhou University, give the answer in the special report on August 29.
Azf's last piece of puzzle for the prevention and control of the epidemic
At present, the global new crown epidemic is repeated, and the proportion of Omikon variants with a strong spread of Omiko has increased, the prevention and control of the epidemic is increasing, and the needs of more therapy and drugs in clinical clinical treatment has further increased. The current research and development and vaccination of vaccines has gradually improved, but the treatment drugs, which are extremely important as an epidemic prevention, are still scarce.
Henan's first 1.1 type 1.1 innovative medicine Azfdin is expected to supplement the last piece of puzzle to prevent and control the epidemic.
Why do you say that? Let's start with Azfdin's pharmacology.

On August 29th, Professor Chang Junbiao, deputy secretary of the Party Committee and vice president of Zhengzhou University, made a special report on the R & D of Azfding at the Ninth Standing Committee Meeting of the Henan Provincial Science and Technology Association.
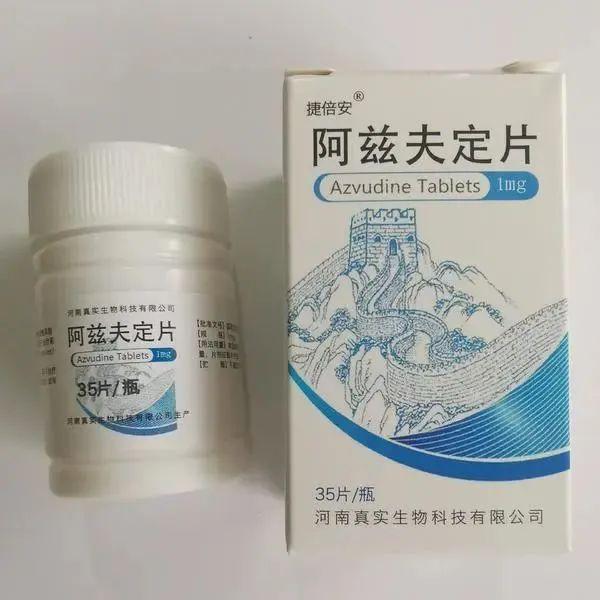
Azf's fixed tablet, as the first anti -AIDS oral oral nucleoside drug with independent intellectual property rights, is also the first domestic new crown pneumonia small molecule oral drugs to be approved for listing.
Azf has the effect of broad -spectrum inhibitory RNA virus replication. Although the new coronal virus is not the same as AIDS virus, it also belongs to the virus of RNA as a genetic material. Azfding, as a nucleoside analog of inhibitory RNA -dependent RNA polymerase (RDRP), can specific effects on the new coronary virus RDRP, thereby inhibiting virus replication, and its drug is targeted and long -term.
"If the RNA virus is replicated to the 'built house', nucleoside (acid) is raw material. Azf will definitely be a 'inferior' raw material, which can trick RNA virus for 'building houses'. It will collapse, so that the RNA virus cannot be copied. "Chang Junbiao vividly described the principle of Azfdin at the scene.
Related experiments show that Azf will have a broad -spectrum to inhibit the replication of RNA virus. The new coronal virus is also an RNA as the virus of the genetic material. It has proven that the drug has a good inhibitory effect on the new coronary virus. At the same time, it is also effective for mutant viruses. As an oral medicine, Azf is convenient for medication and facilitates the use of doctors. In addition, it also has easy production and storage and transportation, which can achieve global distribution.
On August 9, 2022, the General Office of the National Health and Health Commission and the Office of the State Administration of Traditional Chinese Medicine issued a notice saying that the medicine was included in the "New Coronary Virus Pneumonia Diagnosis and Treatment Plan (Ninth Edition)". According to the "diagnosis and treatment plan", Azf's fixed tablet is used to treat adult patients with COVID-19). The usage is swallowed in an empty stomach, 5mg each time, once a day, the course of treatment is not more than 14 days.
At present, Azf's fixed film has been included in the scope of medical insurance in many places in China. According to the price of medical insurance networks in various places, Azf's fixed price is priced at 270 yuan per bottle, 35 tablets per bottle, and 1 mg per piece.
People in the field of pharmaceuticals revealed that the price was only about one -tenth of the prices of similar drugs abroad. Compared with the new crown therapeutic drugs currently on the market, the price of Azf's fixed tablet has a great advantage.
Is Azf the safe?
Is there such a good treatment effect, is Azf's safe? Is there toxic and side effects?
At the meeting, Chang Junbiao also responded to the voice of Azf's settlement on the Internet before.
Top news reporters sorted out and found that in June 2022, in the application for listing technology review report given by the National Drug Administration's Drug Review Center, in the evaluation of drug safety, the AMES test "AMES Test, AMES Test, CHL chromosome distortion test and mouse micro -nuclear test results are positive. " In the reproductive toxicity test, the dose set in the test in the test, which has an impact on the development of rats and rabbit embryos.
Since then, some foreign media have begun to hype on Azfdin's toxic side.
Professor Chang Junbiao said that leaving doses to talk about the toxicity of drugs or the potential adverse risks of drugs is unscientific.
Top news reporters learned that the dose of genetic toxicity and reproductive toxicity in the Azf's scheduled review report is the safety dose for toxic testing on animals, and the treatment dose for clinical human body is two different concepts.
In the AMES test (pollutant caused by pollutants), the test concentration under the safety dose is nearly 100,000 times the concentration of blood concentration of the human clinical dose. In the CHL chromosome distortion test, the drug exposure under the safety dose of reproductive toxicity experiments is more than 60 times the exposure of the clinical treatment dose.
The drug side effect of the drug is directly related to the dose, and the exposure or the concentration of the drug in Azf's clinical treatment dose is far lower than the dose of toxic side effects caused by non -clinical trials.
Professor Cen Xiaobo, director of the National Chengdu New Pharmaceutical Safety Evaluation Center, pointed out in an interview with CCTV that clinically speaking, Azf's genetic toxicity and reproductive toxicity have a large safety window. For "genetic toxicity" and "reproductive toxicity" mentioned in the Azf's final review report, ordinary patients do not need to worry too much. In other words, it is safe to take Azf under the dose of human clinical treatment.
Azfdin will explore the prevention and control in the future. Nowadays, Azf's fixed tablet has been verified that it has the effect of inhibiting the activity of the new coronary virus. Early applications can quickly reduce the virus load and shorten the patient's time to turn the time.
However, in addition to the control of the new crown epidemic, in addition to effective therapeutic drugs, it is also essential to prevent drugs.
At present, there is no new crown drug for those who have been approved for exposure or asymptomatic infected.Internationally, the research and development competition for drugs in the field of new crowns has begun.
Three -phase research after exposure to foreign manufacturers Pfizer has failed, and Meridon has begun to prevent three stages of prevention after exposure.
Chang Junbiao revealed at the scene that due to the long half -life of Azfdin, good efficacy, and small side effects, it has the potential to develop into a new crown prevention medicine.Their team has begun to start the development of drug prevention for Azf.
In the future, Azf is expected to make maggots.
Source: Henan Business Daily
- END -
In August Forest Open Sun Fire Registration

Simple Forest · Psychological Health Center provides you:Children, adolescents, a...
What is the occurrence of NSCLC, which is common and rare target mutations?What new progress?
*For medical professionals for reading referenceOne article clarified!The incidenc...