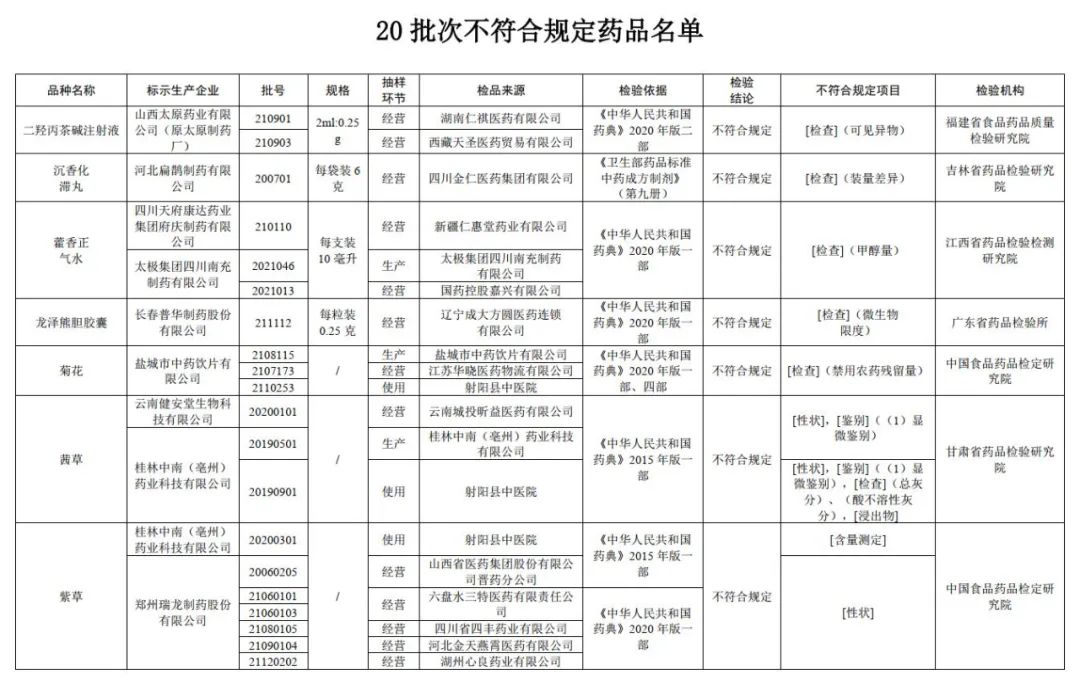
20 batches of medicines are not compliant!Huoxiangzhengqi water, agarwood stagnation pill, etc.
Author:Harbin Daily Time:2022.08.31
The State Drug Administration recently issued a notice that with the inspection of six pharmaceutical inspection institutions including China Food and Drug Inspection and Research Institute, it is marked as the purple grass and Huoxin produced by 9 companies such as Zhengzhou Ruilong Pharmaceutical Co., Ltd. and Tai Chi Group Sichuan Nanchong Pharmaceutical Co., Ltd. 20 batches of drugs such as Xiangzheng Qi Shui are not in line with the regulations.
Screenshot of the official website of the State Drug Administration
According to the notice, the two batches of dihydroxolidal cordonine injection produced by Shanxi Taiyuan Pharmaceutical Co., Ltd. (Yuan Taiyuan Pharmaceutical Factory) marked by the Food and Drug Quality Inspection and Research Institute in Fujian Province did not meet the regulations. foreign body. It is reported that the foreign body refers to the insoluble substances that can be observed under the specified visual conditions, and their particle size or length is usually greater than 50 microns under the specified visual conditions.
After inspection by the Jilin Provincial Academy of Pharmaceutical Inspection and Research, a batch of agarwood stagnation pills produced by Hebei Bianzhang Pharmaceutical Co., Ltd. did not meet the requirements, and did not meet the requirements of the specified project as the difference in volume. It is reported that the difference in volume difference is the indicator of the uniformity of the drug, which is one of the important parameters to ensure accurate administration.
After inspection by Jiangxi Pharmaceutical Inspection and Inspection and Research Institute, it is marked that the three batches of Huoxiang Zhengqi water produced by Sichuan Tianfu Kangda Pharmaceutical Group Mansion Group and Tai Chi Group Sichuan Nanchong Pharmaceutical Co., Ltd. do not meet the requirements, and the project does not meet the regulations. Monol amount. It is reported that the amount of methanol reflects the content of methanol that may be brought by ethanol in ethanol preparations such as alcohol or pyrine.
It was tested by the Guangdong Pharmaceutical Inspection Institute, and a batch of Longze Xiong Bile Capsules produced by Changchun Puhua Pharmaceutical Co., Ltd. did not meet the regulations and did not meet the requirements of the regulations as microbial limits. It is reported that the microbial limitation of microorganisms that are not directly entered the human body to enter the human body. Due to the slightly lower risk of drugs for such drug preparations, a certain number of microorganisms can be allowed, but some conditional pathogenic bacteria shall not be detected.
The three batches of chrysanthemum chrysanthemums produced by Yancheng Traditional Chinese Medicine Drinking Piection Co., Ltd. did not meet the regulations and did not meet the requirements of the regulations as the disabled pesticide residue.
After inspection by the Gansu Provincial Academy of Pharmaceutical Inspection and Research, a batch of Qiancao produced by Yunnan Jian'antang Biotechnology Co., Ltd. does not meet the regulations, and does not meet the requirements of the specified projects including traits and identification; Two batches of Qiancao do not meet the requirements, and do not meet the specified projects include traits, identification, total ash, acid insoluble gray, and immersion.
It is reported that the immersion items in Chinese medicinal materials and drinks can reflect the content of the inner component of Chinese medicinal materials and slices. The irregularity of the origin, growth period, harvest season, processing method, and processing process of Chinese medicinal materials and slices may cause its immersion to not meet the regulations.
After inspection by the China Food and Drug Inspection Research Institute, a batch of purple grass produced by Guilin Zhongnan (Luzhou) Pharmaceutical Technology Co., Ltd. does not meet the regulations and does not meet the requirements of the specified project as a measurement. The six batches of purple grass do not meet the regulations and do not meet the requirements of the specified projects.
It is reported that the appearance, odor, taste, solubility, and physical constant are recorded under the traits, which reflect the quality characteristics of the medicine to a certain extent. Traditional Chinese medicines do not meet the regulations, and may involve the deviation of the species of medicinal materials, defects, and improper storage of the processing process.
The State Drug Administration stated that the drug supervision and management department has required relevant enterprises and units to take risk control measures such as suspension of sales and recalls, and conduct investigations and rectify rectification on the reasons of not compliance with the prescribed reasons.
In addition, the State Drug Administration requires relevant provincial drug supervision and management departments to organize investigations on suspected illegal acts existing above -mentioned enterprises and units in accordance with the Drug Administration Law of the People's Republic of China, and publicize the results of the results in accordance with regulations.
Source: People's Daily Online

PSAs

- END -
Small disease causes major problems, and nose bleeding can't stop 4 days!
The hot and dry air in summer can easily lead to the expansion and rupture of the ...
From now on, Qiantang District, Hangzhou, the 7 -day test of negative proof or nucleic acid detection certificate!
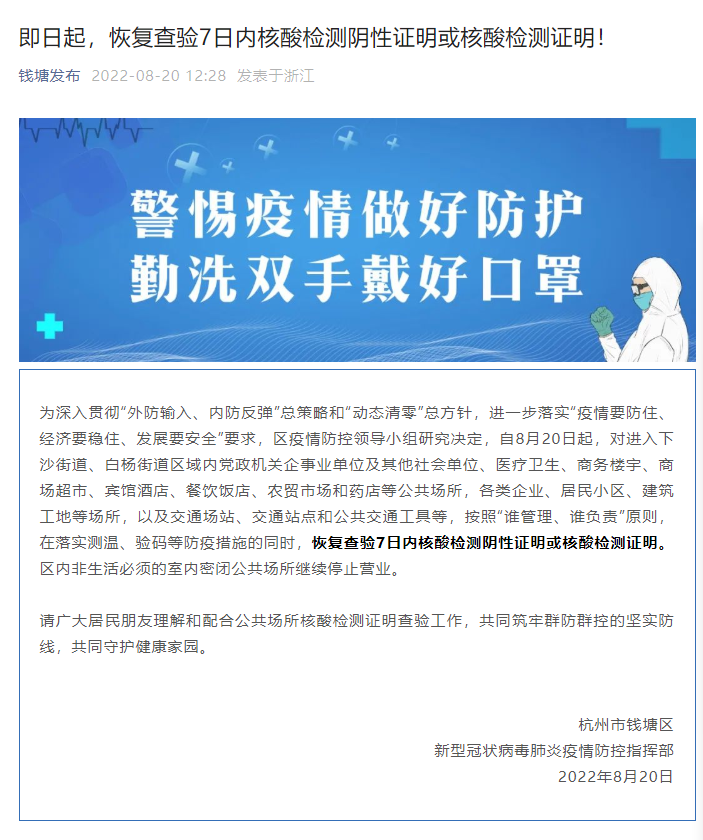
This afternoon, the new type of coronary virus pneumonia in Hangzhou Qiantang Dist...