Newly made new progress in lung cancer!Which patients can benefit?| 2022 WCLC
Author:Cancer Channel of the Medical Time:2022.08.30
For medical professionals for reading reference

Come and learn!
As immune and targeted therapy changed the pattern of lung cancer diagnosis and treatment, neoading immunity and targeted therapy have achieved great development in the field of non -small cell lung cancer (NSCLC). In 2022, the International Cancer Research Association (IASLC) World Lung Cancer Conference (WCLC) came to an end.
Summary:
1. NADIM II Studies are updated! Neo -assisted combined treatment brings survival benefits for removal IIIA/B NSCLC!
2. How to find out high progressive risk patients in NADIM tests in NADIM tests? Tumor Bulk-RNA SEQ test!
3.Togather and better! Tripley Mippling+Platinum Double -Containing New Assistance Treatment Potential Removal NSCLC is safe and efficient!
4. Irae foreshadow the opportunity of "rebirth"? Tripley Mippling+Platinum Double-Containing Dipper Chemotherapy New Auxiliary Treatment Iib-IIIB Pharmaceutical NSCLC Renaissance Research New Research
5.EGFR mutation IIIA phase NSCLC new assisted Chinese "answer" for joint treatment! ANSWER Research: Amerinib vs Elotinib
6. "First HER2+NSCLC Drug" entered the field of NSCLC new assisted therapy! A practical case of intuitive "magic medicine" effect!
Progression Free Survival and Overall Survival in Nadim II Study
INadim II Study: No Progress Survival (PFS) and General Survival (OS) results
(Summary number: PL03.12)
In the NADIM test, neo -assisted combination treatment has been proven to be very effective for removal NSCLC. The conference updated the results of the NADIM II random test.
NADIM II (NCT03838159) is an open label, random, arms, phase II, multi -center clinical trials. ECOG PS 0-1, and there is no known EGFR/ALK mutation period (according to AJCC 7th edition) NSCLC patients, randomly divided into two groups at a ratio of 2: 1: the joint treatment group accepted Nawu Liyou Mippling 360mg+paclitaxel 200mg/m2+card platinum AUC 5, every 21 days (+/- 3 days) is 1 cycle, a total of 3 cycles as new auxiliary treatment; separate chemotherapy group receives 200 mg/m2+card platinum AUC 5, every 21 days (+++every 21 days (++ /-3 days) is 1 cycle, a total of 3 cycles; subsequent surgery. Patients with R0 resection confirmed by the pathology evaluation of the pathology began the 3rd to 8 weeks (+7 days) after surgery. The main endpoint is the pathological alleviating (PCR), and the secondary end points include PFS, OS, and biomarkers analysis.
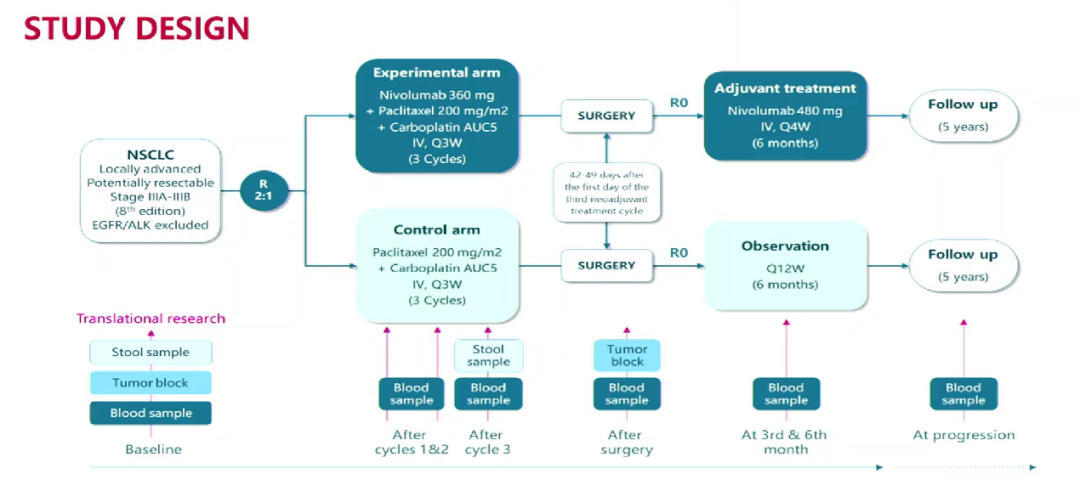
Test design
The results showed that 93.0%and 69.0%of patients were performed in the combined treatment group and separate chemotherapy group for final operations. Among all patients, 11.0%of patients underwent general pulmonary dehumidification, 78.1%underwent pulmonary lobe destruction, 6.8%underwent bilateral lobe removal, 2.7%underwent pulmonary segmentation, 1.4%underwent right lower lobe resection And lung segment resection. 92.5%of patients in the combination group underwent R0 removal, and the single chemotherapy group was 65.0%.
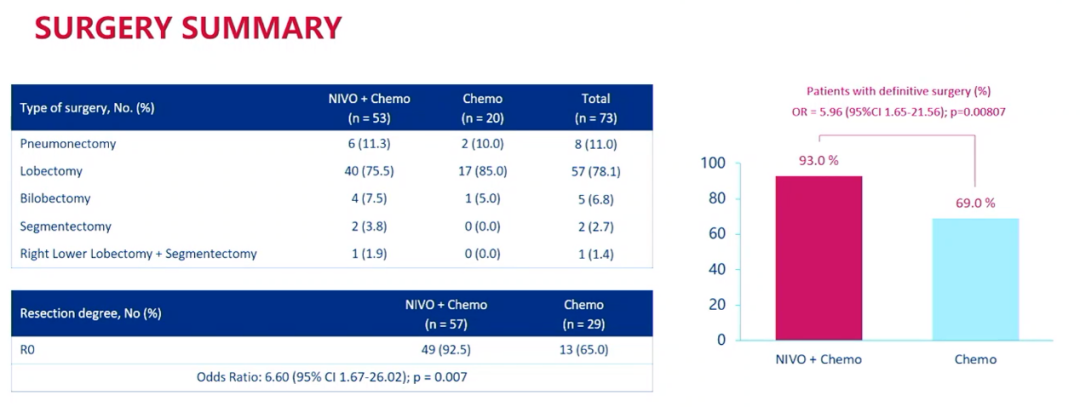
Overview of surgery
In addition, the downgrade of the combined treatment group was 69.8%, while the separate chemotherapy group was 40.0%.
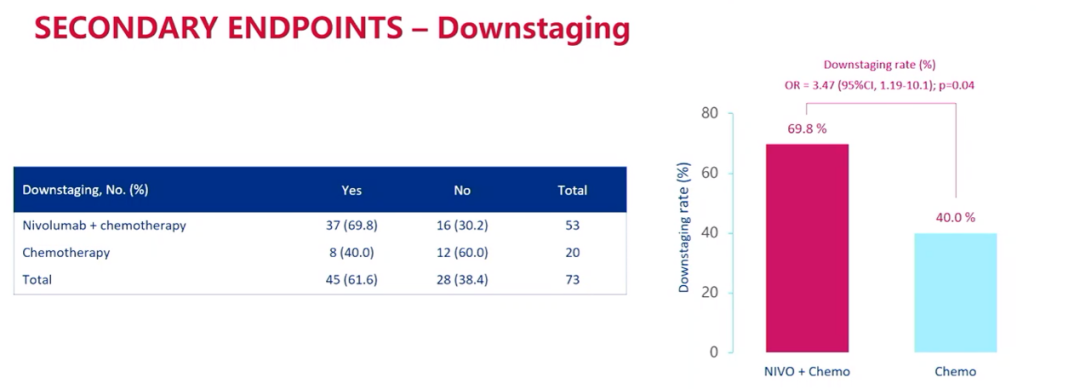
Downgrade data
After the median follow -up of 26.1 months, the 24 -month PFS rates of the combined treatment group and the individual chemotherapy group were 66.6%and 42.3%, respectively. The combined treatment group did not reach the median PFS, while the median PFS of the separate chemotherapy group was 18.3 months, (HR = 0.48; 95%CI: 0.25-0.91; P = 0.025).
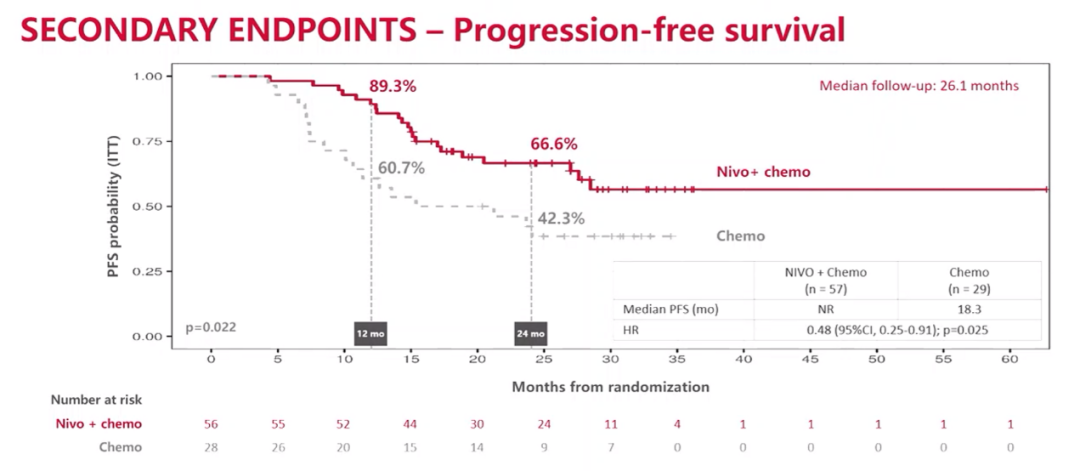
PFS result
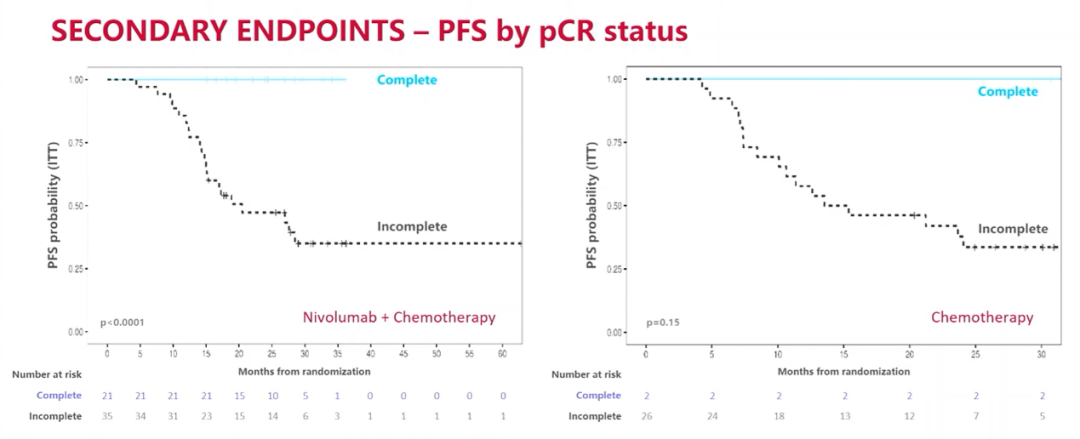
Based on the analysis of PFS according to the PCR status
The 24-month OS rates of the combined treatment group and separate chemotherapy group were 84.7%and 63.4%, respectively, and neither of the two groups reached the median OS (HR = 0.40; 95%CI 0.17-0.93; P = 0.034).
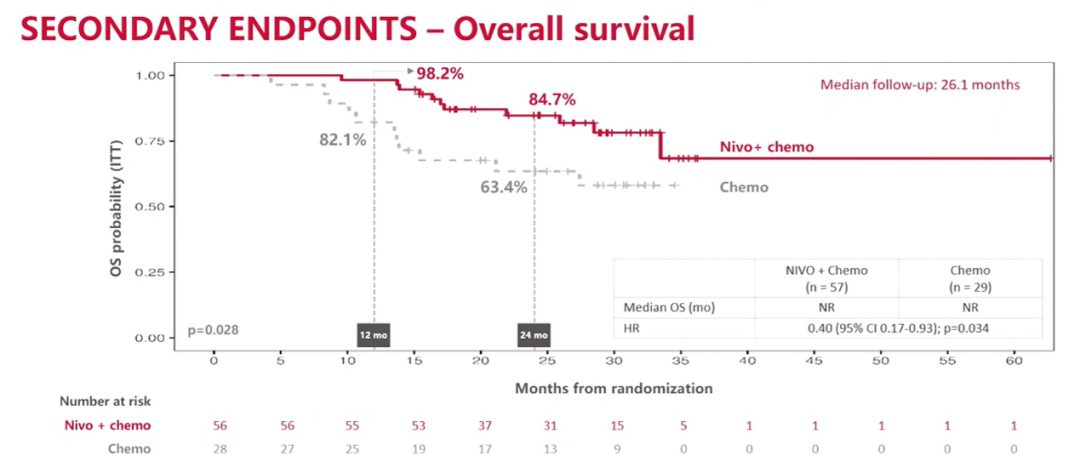
OS result
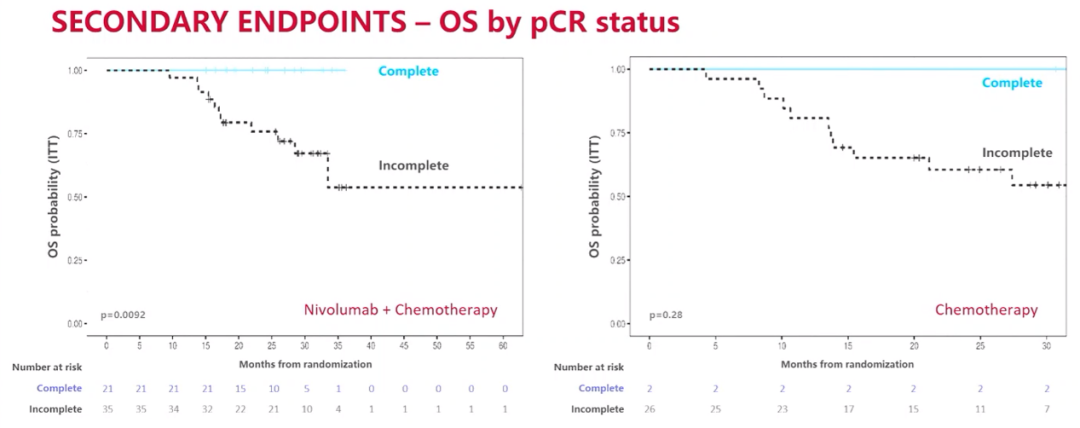
According to the analysis of OS according to the PCR status
The latest survival data of NADIM LL research confirms the curative effect of NSCLC in NSCLC in the NSCLC -combined platinum chemotherapy neo -assisted treatment. This benefit is particularly obvious among patients with positive expression and realization of PCR. NADIM II research is also the first clinical study of OS benefits to patients with a combination of combined neo-assisted therapy in patients with removal IIIA-B phase NSCLC.
TUMOR BULK-RNA SEQ Identifies Patients at High Risk of Progression in Non-Complete Pathology Responders from Nadim Tric
K Tumor Bulk-RNA SEQ detection can identify patients with a total pathological relief in NADIM tests in the NADIM test (Abstract No.: OA02.03)
Pathological relief is considered an alternative indicator of the survival of patients with new assisted immunotherapy. In this sense, the risk of disease progress in patients with non-complete pathological relief (NON-CPR, non-CPR) patients is higher than those of patients with complete pathological relief (CPR). In order to determine the genetic expression mode that may affect the long -term prognosis of this high -risk population, this study has detected the surgical specimen of non -CPR patients, and analyzes the differences between progressors and non -progressives.
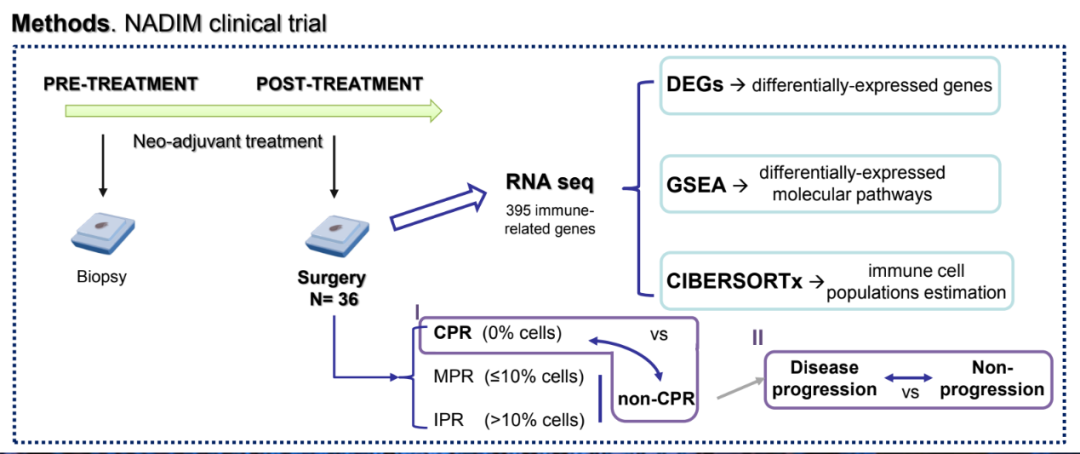
Researchers analyzed the samples of surgical tissue samples in the NADIM test that can remove the IIIA phase NSCLC patients. Using ONCOMINE Immune Response Panel, which is based on 395 genes related to genes, sequencies for tumor RNA. Use DESEQ2 and Gene Set Enrichment Analysis (GSEA) assessment groups (DEG) and pathway enrichment analysis, and use the CIBERSORTX method to estimate the proportion of immune cell subtypes. Patients are divided into two groups: CPR (n = 22) and non -CPR (n = 14). Depending on whether there is a disease progress in 34.2 months after the diagnosis, non -CPR patients are further divided into progressives (p, n = 5) or non -progressive (np, n = 9). Based on the ROC curve analysis, the maximum value of the seemingly ratio is the threshold, and the DEG or immune cell sub -group of each patient is divided into high or low group.
Test design
The results showed that there were 22 gene expression among non -CPR patients compared with CPR patients, of which most of the genes and proliferations (CDKN3, CCNB2, Kiaa0101, MKI67, BUB1, CDK1, TOP2A, Foxm1, MAD2L1), tumor logo Things (CDKN2A, KRT5, BRCA1, Twist1) and other (Magea3, CEACAM1, CXCL8, TNFRSF18, G6PD, HMBS, DGAT2, ISG15).
CPR and non -CPR patient genetic expression differences
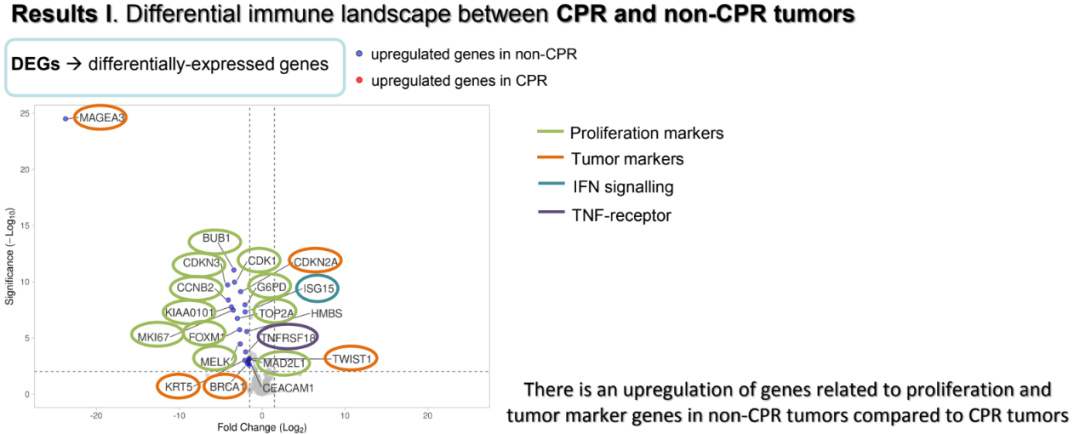
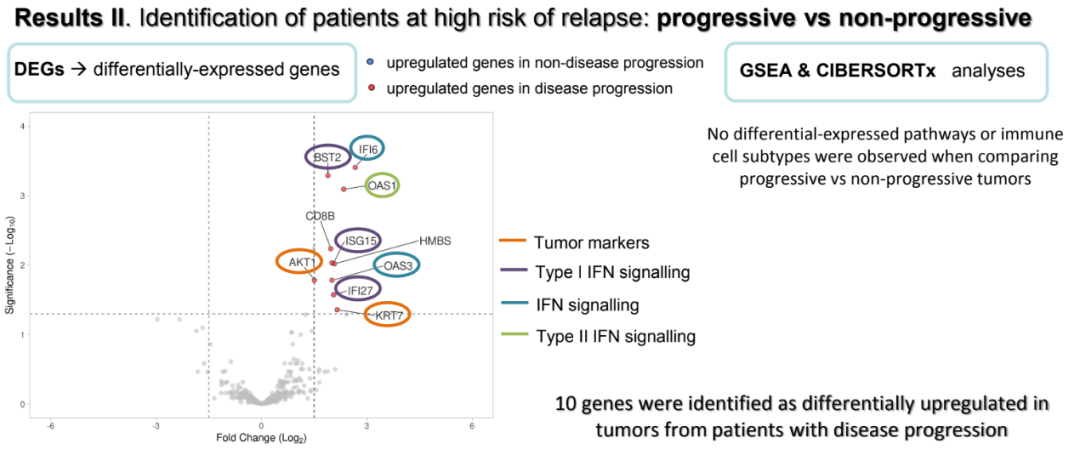
Further GSEA analysis shows that among CPR patients, the pathogens related to antigen processing, TCR expression, and lymphocyte infiltration are adjusted. Patients who are not CPR show the upward adjustment of proliferation, tumor logo, interferon, housekeeper gene and tumor antigen signal -related pathways. In terms of the differences between P and NP, 10 gene differences among P and NP patients: IFI6 and OAS3 participating in the interferon signal transduction; AKT and KRT7 as tumor logo; ISG15 and IFI27 and CD8B, SDHA, HMBS and OAS1.
The genetic expression difference between progressive and non -progressive (10 genes)
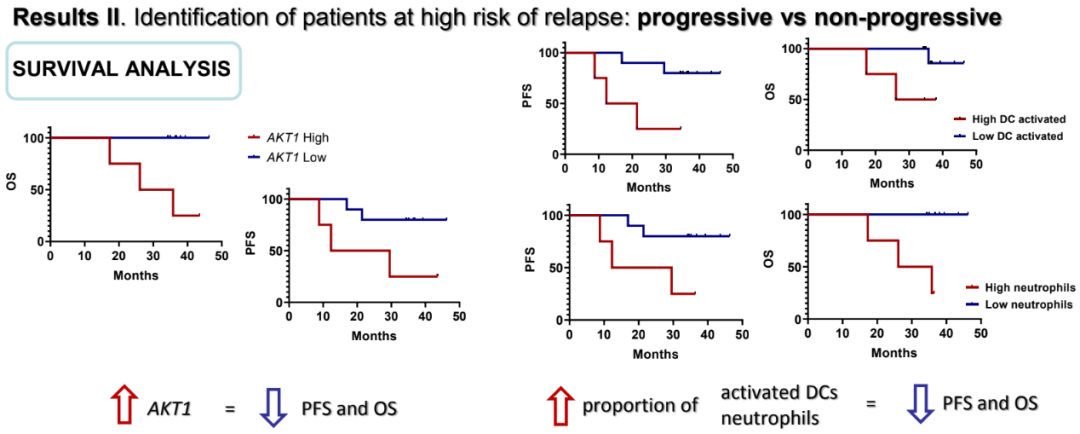
Non -CPR patients with higher levels of IFI6 (P = 0.010), BST2 (P = 0.010), CD8B (P = 0.019), AKT (P = 0.033), OAS3 (P = 0.010) and IFI27 (P = 0.010) related to short PFS. In addition, higher HMBS (P = 0.018) and AKT (P = 0.003) level are related to short OS. Patients expressed in high AKT (HR = 10.31; 95%CI 1.2-88.8) and death (HR = 50.6; 95%CI 3.77-680.5) risks are significantly higher. High AKT expressing the median PFS of patients is 12.3 months (95%CI 0-32.6), while the median PFS of low-expression patients has not been reached. There are no differences in the proportion of cells in P and NP patients. Instead of the activation ratio of trees and cellular cells in non -CPR patients, PFS (P = 0.019, P = 0.053) and OS (P = 0.033, P = 0.003) are low.
Progressive and non -progressive characteristic survival analysis
Studies have shown that the expression of the expression of surgical specimens related to CPR patients shows that the treatment of PD-1 inhibitors can produce an effective immune response. In addition, immune expression characteristics related to disease progress found in the surgical specimen of non -CPR patients may help follow up and manage these high -risk patients.
An UPDated Analysis of Toripalimab and Platinum-Double CHEMOTHERAPY As Neoadjuvant therapy for Potentially Resectable NSCLC
联Traipley Mippling combined with platinum dual drug chemotherapy new assisted therapy for potential removal of NSCLC patients (TOGATHER Research)
(Summary number: 2290/EP05.02-021)
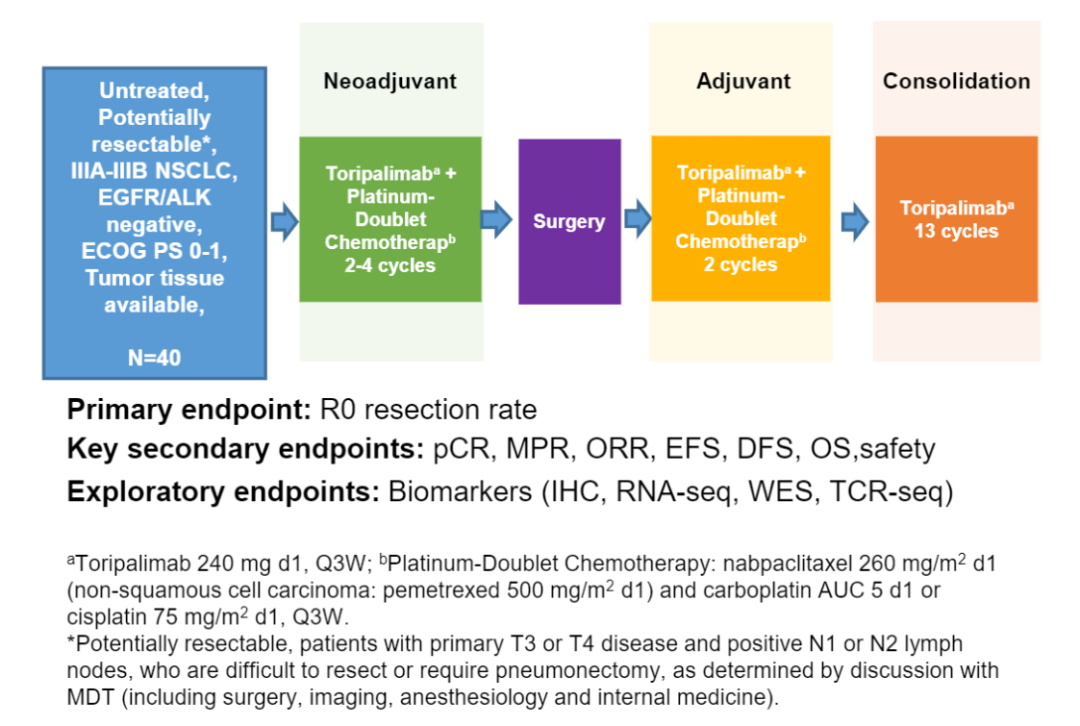
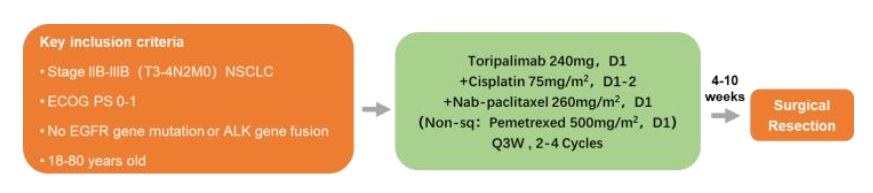
PD-1/PD-L1 inhibitors are treated as neo-assisted therapy in patients with non-small cell lung cancer (NSCLC) in the IB-IIIA. Phase III NSCLC is highly heterogeneous, and clinical treatment plans are still controversial. Study of this forward -looking, single -arm, and II phase is designed to explore the efficacy, safety, and feasibility of Tripley combined with platinum -containing dual drug chemotherapy for potential removal NSCLC initial treatment. The preliminary results of this study were announced in 2021 WCLC, and the update data was announced at the meeting. The study incorporated a non-drive-gene mutation IIIA-IIIB potentially removed NSCLC patients. All patients accepted 2-4 cycles of Tripley Midarity (240 mg D1), albumin paclitaxel (260 mg/m2D1) (non-squamous cell carcinoma: Pemeusus 500mg/M2D1) and Card platinum (AUC 5 d1) or cisplatin (75mg/m2D1), once every 3 times. New assisted treatment is performed within 3-5 weeks after the completion of the surgery evaluation and surgical indication evaluation. Within 30 days after surgery, the auxiliary Tripley Mipide was given 2 cycles of chemotherapy, and then 13 cycles (240 mg, Q3W) Tripley single anti -single drug treatment was given. The main endpoint is R0 resection rate. The secondary end point is PCR, main pathological relief (MPR), disease -free survival (DFS) and safety.
Togather research design
As of March 15, 2022, a total of 40 patients with Phase III had potentially removed NSCLC patients, and 7 of them were still undergoing new assisted treatment. Among the 33 patients can be evaluated, the median age is 58 years old, 12.1%is female, and 18.2%is non -smoking. Simpanocyte lung cancer accounts for 87.9%(29/33) and 45.5%(15/33).
Can evaluate patient baseline characteristics
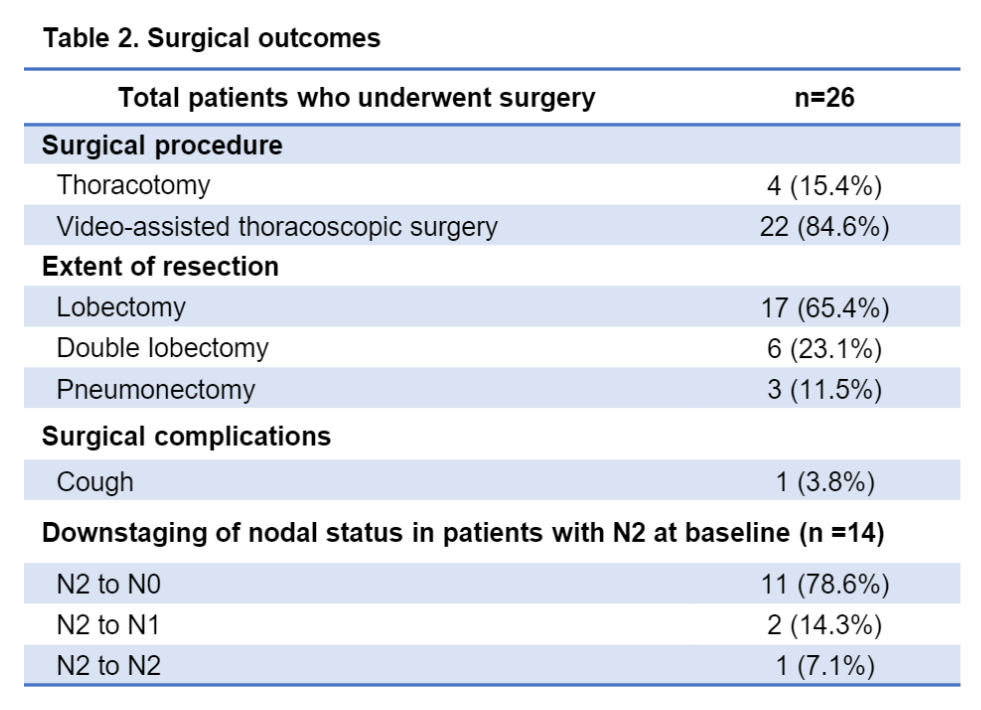
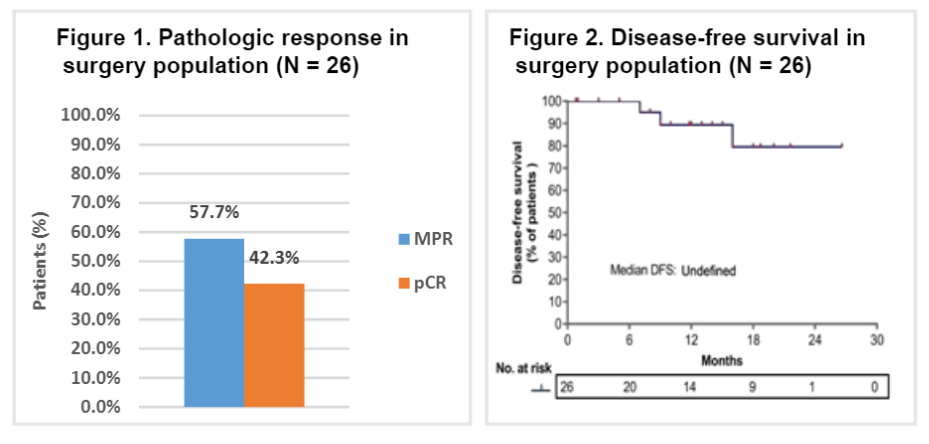
Among the evaluated patients, 30 (90.9%) meet the surgical standards. Four patients rejected surgery for economic and personal reasons. All 26 patients undergoing surgery achieved R0 resection. The actual surgery rate is 78.8%(26/33), MPR is 57.7%(15/26), and PCR is 42.3%(11/26). In the surgery group, the median follow-up time was 16 months (scope: 3-28), and the DFS rates of 1 and 2 years were 89.4%and 72.9%, respectively. No new non -expected adverse events are observed.
Surgical result
PCR and DFS data of the surgery population
The update result has further proved that Tripley Mippy is highly efficient and good tolerance. More importantly, the expected survival rate is expected to increase.
Neoadjuvant TORIPALIMAB Combination in Patients with Stage Iib-IIIB NSCLC: A SINGLE-RM, PHASE 2 TRIAL (Renaissance Study)
Tripley combined with platinum dual drug chemotherapy new assisted therapy IIB-IIIB phase NSCLC: a stage II, single-arm research (Renaissance Research)
(Summary number: WS08.22)
PD-1 inhibitors have shown potential anti-tumor activity in NSCLC. This study is a single center, with a forward-looking study, which aims to explore the efficacy and security of Terpt Midurate combined with platinum-containing dual-electrocroofing new assisted therapy for the treatment of Iib-IIIB phase NSCLC.
This study includes the IIB-IIIB stage, EGFR/ALK wild type, ECOG PS 0-1 NSCLC patients. Patients received 2-4 cycles of Tripley Midarity (240mg, Q3W) combined with neo-auxiliary treatment of platinum-containing dual pharmaceutical chemotherapy, and received imaging/surgical indications after treatment in the second cycle. Patients who cannot perform surgery will re-evaluate after an additional 1-2 cycle neo-assisted treatment. The main endpoints are MPR and PCR. The secondary end point is the objective relief rate (ORR), R0 resection and safety.
Test design
Since March 2021, a total of 53 patients (median age: 62 years old, IQR: 45-76; women: 5,9.4%; squamous cell carcinoma: 42, 79.2%) were included in research, and 2- New assisted treatment of 4 cycles. Among them, patients with IIB, IIIA, and IIIB were 15, 30, and 8, respectively. Fifteen cases are in the pre -surgery or are not suitable for surgery.
Patient baseline data
39 patients underwent surgery (new assisted treatment and medium interval between surgery: 67 days, IQR: 39-113). 25 cases (25/39, 64.1%) obtained MPR, of which 20 (20/39, 51.3%) achieved PCR. All 39 cases (100%) achieved R0 resection. 29 patients (29/31, 93.5%) patients underwent CN2/N1 surgery during the base line, achieving the lymphatic reduction period. 49 patients completed the treatment plan and imaging review, and ORR was 85.7%(42/49).
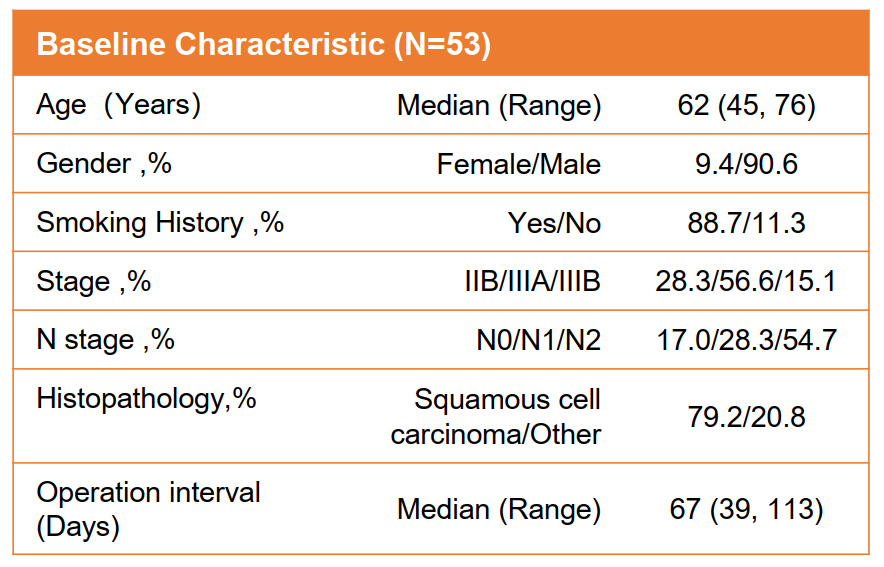
Image alleviation data
There were 46 patients (46/49, 93.9%) and 15 patients (15/49, 30.6%) patients reported level 1-2 and 3-4 Traes. Among the patients who received surgery, three patients (3/39, 7.7%) underwent 2-3 Irae (enteritis or rash) and received glucocorticoid therapy. Interestingly, these three patients have achieved PCR, and the mid-range interval between neo-assisted therapy and surgery is 56 days (IQR: 56-70). Compared with all patients undergoing surgery, these three patients did not treat related surgical delays and extra surgery. This also indicates that the PCR rate of patients with level 2-3 IRAE is better (100%than 47.2%). Among the patients who are preparing for surgery or are not suitable for surgery, two patients experienced level 2-3 IRAE (enteritis or pneumonia) and received glycopal hormone treatment. After 10 months of observation, keep the condition stable (SD); the other case is fully clinically relieved (CCR) after receiving neo -assisted treatment.

Of all patients who have experienced Irae and received glucocorticoid treatment, Irae's disease time is 12 days (IQR: 7-54), and the median duration of glucocorticoid therapy is 12 days (IQR: 7-79 To.
Patient IRAE data
The results showed that Tripley combined with platinum dual drug chemotherapy new assisted therapy for the treatment of Iib-IIIB phase NSCLC patients with significant effects, safety tolerance, and application prospects. Grade 2-3 IRAE has the effect of the plan or potential correlation.
AUMOLERTINIB Versus Erlotinib/Chemotherapy for Neoadjuvant Treatment of Stage IIIA EGFR-Mutant NSCLC (ANSWER)
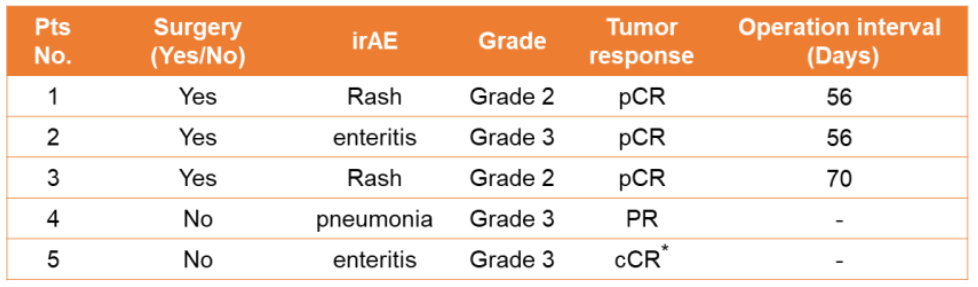
阿AnWER Research: Amerininib compared to EGFR mutations NSCLC new assisted therapy for EGFR mutations in IIIA phase IIIA
(Summary number: EP05.02-009)
For the NSCLC of the IIIA-N2 phase EGFR mutation, a number of random control studies have compared various EGFR-TKI targeted therapy for combined chemotherapy as new assisted therapy. However, the role of the third-generation EGFR-TKIS in the new auxiliary environment has not yet been concluded. Ameitinib is a new third -generation EGFR TKI, which is approved by NSCLC for the treatment of EGFR mutations in China. ANSWER Studies will evaluate the efficacy and security of Ameitininib and Elotininib/Double Platinum Chemotherapy (Bemetatga Platinum or Cisplatin) newly -available EGFR mutations.
Test design
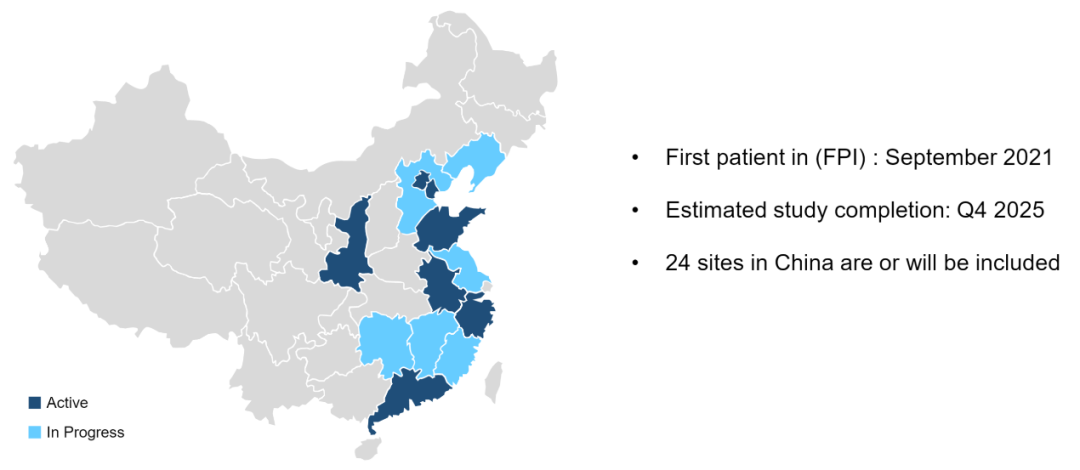
This is a multi -center, open label, random control test. The previous IIIA-N2-stage non-scale NSCLC patients who have not been treated and historians have confirmed or potentially removed, and they carry sensitive EGFR mutations, which meet the requirements of this research. About 168 patients will be randomly (1: 1) into the Ameitininib group (group A) or Eloatinib/chemotherapy group (group B), and layer layered according to EGFR mutations (EX19DEL VS.L858R). Patients in group A orally Ameitinib 110 mg/d (new assisted therapy, 42D; auxiliary treatment, up to 12 months), Group B patients orally Elotinini 150 mg/d , For up to 12 months)+Pemeuscern 500 mg/m2+card platinum AUC 5 or cisplatin 75mg/m2 (new assisted treatment, 2 cycles; auxiliary treatment 2 cycles). The main end point is ORR. The secondary end points include PCR, MPR, DFS, no event survival (EFS), OS, R0 resection rate, cutting rate and safety. Adverse reactions are classified by CTCAE V.4.0. The first patient joined the group in September 2021. It is expected to be completed in Q4, 2025. Looking forward to the good news of the Chinese team!
Test admission group
Neoadjuvant DS-8201 for Stage III NON-Small Cell LUNG CANCER with Her2 20ins
-New Auxiliary DS-8201 in the efficacy of patients with NSCLC patients in HER2 20IN III
(Summary number: EP05.02-006)
III-N2 NSCLC is a highly heterogeneous disease that requires multi-mode treatment. When the current period of surgery is difficult to achieve the purpose of tumor reduction, new assisted treatment is required. Trastuzumab DeruxTecan (DS-8201) is a new type of antibody-drug puppet (ADC). Four types of topological areas I inhibitors are connected to HER2 targeted antibodies. Previous studies have shown that in the previously treated HER2 mutant NSCLC, ORR is 55%and has a lasting effect. However, the efficacy and security of DS-8201 in early and late NSCLC patients have not been studied.
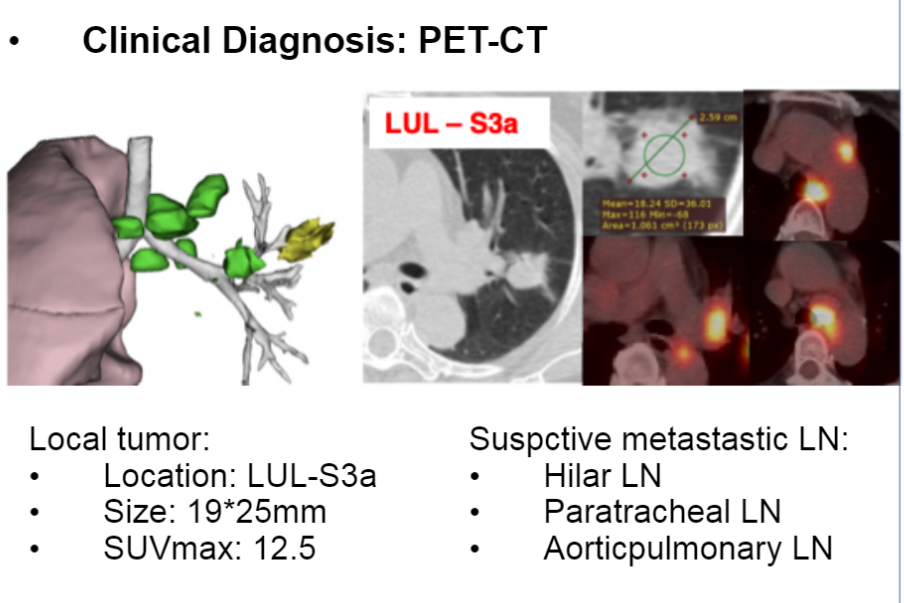
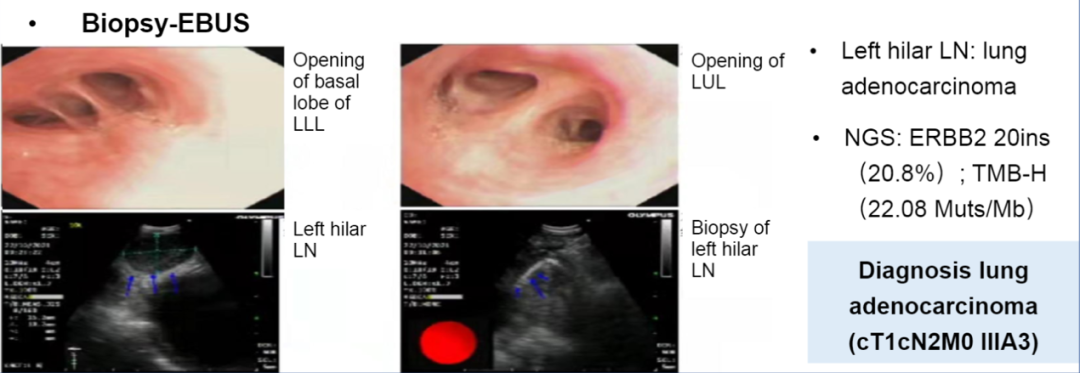
This study reported a patient with a 20INS IIIA phase of NSCLC, which received a three-cycle new assisted DS-8201 (345mg Q3W) treatment, and followed the R0 surgical resection. PET-CT and brain MR examinations are performed before and after treatment. According to the RECIST 1.1 version, the radioactive response was evaluated by two independent doctors, and two independent pathologists evaluated and reviewed the pathological response. Record side effects and surgical results. Patients, women, 58 years old, never smoke, diagnosed with pulmonary adenocarcinoma in the IIIA stage (CT1CN2M0). There was no tumor -related symptoms at our hospital, (ECOG) PS 0. Before the treatment, the PET-CT showed a solid nodule on the upper left lobe. The lower side of the trachea, under the aorta, and the pneumon lymph nodes were enlargement, and the glucose intake increased.
Diagnostic information
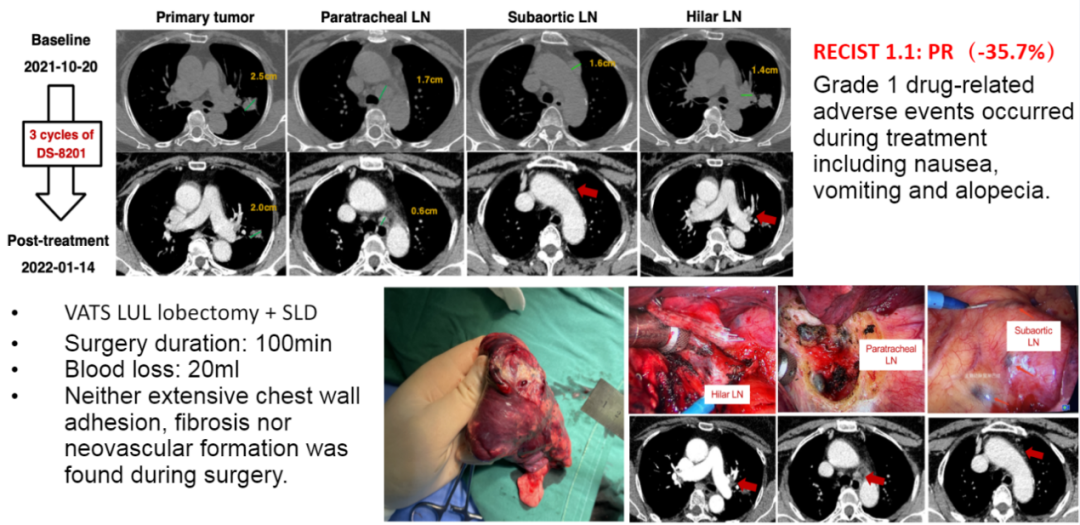
Considering the potential cutability of HER2 mutant NSCLC and the lack of the current lack of optimal treatment, the multidisciplinary committee recommends local intervention after the treatment of DS-8201. After three cycles of treatment, part of it is relieved. Observed the alleviated heterogeneousness between the primary and metastatic lesions, the primary tumor was reduced by 25%, and the metastatic lymph -shrunk was reduced by 65%. After the last dose of DS-8201, the upper left leaf removal was performed and a systemic lymph node cleaning was performed to achieve fully resection (R0). The surgery lasted for 100 minutes, and blood loss was 20ml during operation. No extensive chest wall adhesion, fibrosis and new blood vessels were found during the operation. He was discharged 3 days after surgery, and complications occurred after no surgery.
Healing
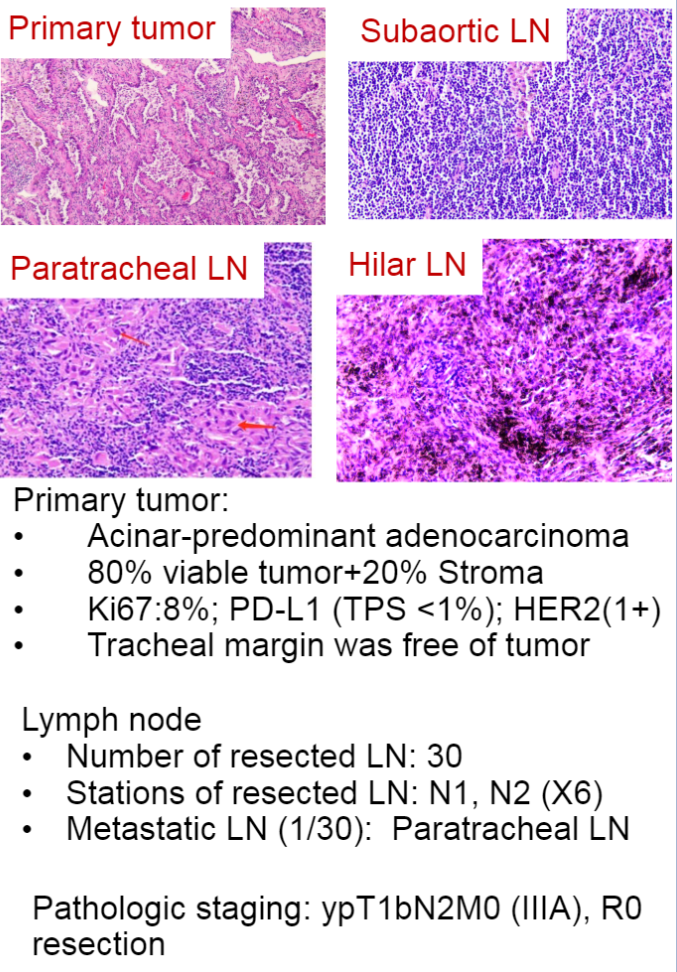
System pathological assessment shows 80%of residual living tumor cells. Except for the pneumatic lymph nodes, the removed lymph nodes have no cancer cells. During the treatment, nausea, vomiting, and hair loss related adverse incidents. Immunohistochemical (IHC) detection prompt CD4 (10%), CD8 (10%), CD20 (10%), CD163 (30%), CD38 (10%) cell infiltration. Her2 protein expression (IHC 1+) after immunohistochemistry, PD-L1 expression negative (22C3) (TPS <1%). Analysis of peripheral blood shows that the treatment of T -cell library diversity.
Pathological results details
DS-8201 has been proven to have anti-cancer activity in local advanced NSCLC, with small side effects and no delay surgery. Regardless of Her-2 protein expression, DS-8201 can play a role. This case provides an in -depth understanding of the potential applications of ADC in new assisted therapy.
Expert Reviews
Neo -assisted immunotherapy is the focus section of WCLC this year. The meeting reported the results of a number of heavy clinical research and logo research, which caused great attention and enthusiastic discussions. Among them, the latest survival data of NADIM LL research confirms the efficiency and good security of the new assisted union, and the Tripley monetta is reported by similar rigorous design. In this year's data follow -up update, it has also been reported. The efficacy and safety, which makes the application prospects of the combined combination model in the treatment of new auxiliary treatment of lung cancer. In the relevant logo research, it is found that the abnormal expression of the RNA abnormality of the tumor gene is not good, and the adverse reaction of immune -related reactions may be a predictive factors for the benefit of the joint treatment. for reference.
In addition, the treatment of new assisted therapy for driving gene -positive patients has also attracted attention. This year's meeting reported a head -to -head -header study (Aceninib vs Elotininib) design in EGFR mutant patients, as well as一项针对HER-2突变肺癌患者采用DS-8201新辅助治疗的病例报道,虽然这些治疗方案尚缺乏有力的研究数据支撑,但有理由相信这类治疗模式有望进一步优化肺癌的多学科管理,进一步Improve the prognosis of patients.
Expert Introduction
Holy Emperor
Doctor of Medicine, Deputy Chief Physician, Cancer Hospital Affiliated to Fudan University
Assistant Director of the Department of Internal Medicine of chest oncology
American Clinical Oncology Society (ASCO) member

Member of the China Anti -Cancer Association Clinical Oncology Chemotherapy Committee
Secretary of COE of the China Pharmaceutical Education Association of the Cycies
China Clinical Oncology Society (CSCO) member
Member of Shanghai Anti -Cancer Association
Standing Committee Member of the Shanghai Anti -Cancer Association CRPC Youth Committee
The internal medicine treatment and research of chest tumors such as lung cancer and esophageal cancer, in depth research in the fields of new target identification, molecular logo research, tumor palliative treatment, etc. have published more than ten SCI papers and presided over 2 scientific research funds.
The first release of this article: the medical world tumor channel
Author of this article: lily
Editor in charge: Sweet
- END -
Summer cardiovascular disease is also high, so don't take it lightly "heart"

In everyone's impression, winter is the high incidence of cardiovascular disease. ...
Rescue of life and death!Jingzhou premature babies were born three days of birth, and the stomach rupture, Wuhan experts surgery overnight to rescue small lives
Jimu Journalist Liu XunCorrespondent Xue YuanA premature infant with a weight of o...