The biomarker of "grounding gas" helps to treat lung cancer treatment!| 2022 WCLC
Author:Cancer Channel of the Medical Time:2022.08.29
For medical professionals for reading reference

2022 WCLC wonderful interpretation
In 2022, the International Cancer Research Association (IASLC) World Lung Cancer Conference (WCLC) was held in an offline+online form from August 6th to 9th. WCLC is an annual ceremony in the field of lung cancer. The results of many heavy research have been announced and promoted clinical practice updates, attracting the attention of experts and scholars around the world.
With the development of testing technology and the emergence of various new drugs, clinicians no longer "go to black" in the treatment, but "adjusted while treating while treating while treating". treat. What new "road signs" bring us this conference? Look at it together!
Summary:
1. How to break the problem of immune and drug resistance, choose the best backline solution of NSCLC? Biomagonist is here!
2. New assisted treatment results early prediction? The CTDNA level of the baseline is significantly related to the OS and PFS of the NADIM II test!
3. Can lung cancer treatment results with a ruler+grip meter? Nutritional intervention needs attention!

Scan the two -dimensional code above, get WCLC cutting -edge information and expert views
HUDSON: An Open-Label, Multi-Drug, Biomarker-Direct Phase 2 Study in NSCLC Patients Who ProgresEd on Anti-PD- (L) 1 Therapy
一Hudson: A phase II study of open labels, multi-drug, and biomarkers guidance for PD-(L) 1 of PD- (L) 1
(Summary number: OA15.05)
HUDSON is a non-small cells that have received platinum dual drug chemotherapy and anti-PD- (L) 1 immunotherapy (single medicine or combination) and in advanced non-small cells after treatment of PD- (L) 1 Multi -arm and umbrella studies carried out among patients with lung cancer (NSCLC). The study evaluated the efficacy, safety, and tolerance of a variety of treatment combinations that changed the customized treatment through molecular changes, and aimed to overcome the resistance to PD-(L) 1 inhibitors. The conference announced the mature efficacy and safety results of the initial joint plan, including the Parp inhibitor (module 1), Danvatirsen (module 2), Ceralasertib (ATR Inhibitors; module 3) and Oleclumab (anti -CD73 antibody; module 5).
According to tumor molecular characteristics, patients are incorporated into the biomarker matching (group A) or non -matching (group B) queue. Group B is further subdivided by the primary or obtained resistance of previous anti-PD-(L) 1 treatment, which is defined as a disease progress in the starting treatment ≤ 24 weeks or after the starting treatment. Based on the total relief rate (ORR)), non -progressive survival (PFS), and overall survival (OS) as the basis to determine whether the amount of each initial queue sample was expanded from 20 to 40 patients. Continuous monitoring safety.

Test design
From January 26, 2018 to April 14, 2018, a total of 255 patients were included in research, and Diaguli Mipida combined with Orapar (N = 87), Danvatirsen (N = 45), Cerasertib (N = 66; Group A queue is accumulated, this report is n = 21) or Oleclumab (n = 57) for treatment. The baseline population statistics and disease characteristics of each treatment group are generally balanced; 41.4%, 44.4%, 50.0%, and 43.9%of patients have previously received ≥3 treatment schemes, 19.5%, 13.3%, 24.2%and 21.1%of the treatment. Patients have received ≥2 platinum -containing therapy.
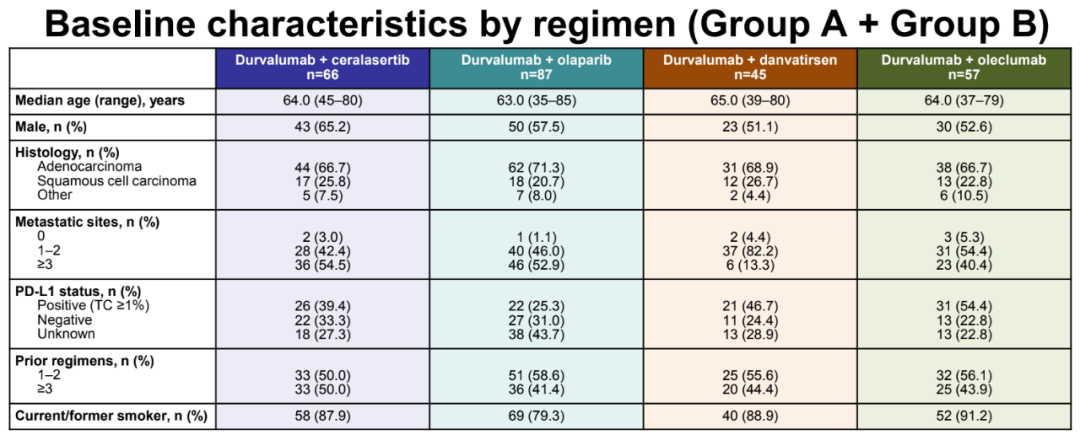
Patient baseline data
In the scheme that has so far, the ORR (16.7%VS 0-4.8%) and 12 weeks/24 weeks of the disease control rate of the ORR (16.7%VS 0-4.8%) of the Cerasertib scheme (12 weeks: 60.6%vs 26.7%-36.8%; 24 weeks: 42.4%VS 13.3%-17.2%) is the highest in value, and the median PFS (6.0 months, 80%CI: 4.6-7.5; other solutions: 1.8-2.9) and OS (15.9 months, 80%CI CI, 80%CI : 14.1-20.3; other solutions: 7.9-11.0) The most longest in value; in this module, the effect of biomarkers matching queues is also the best.
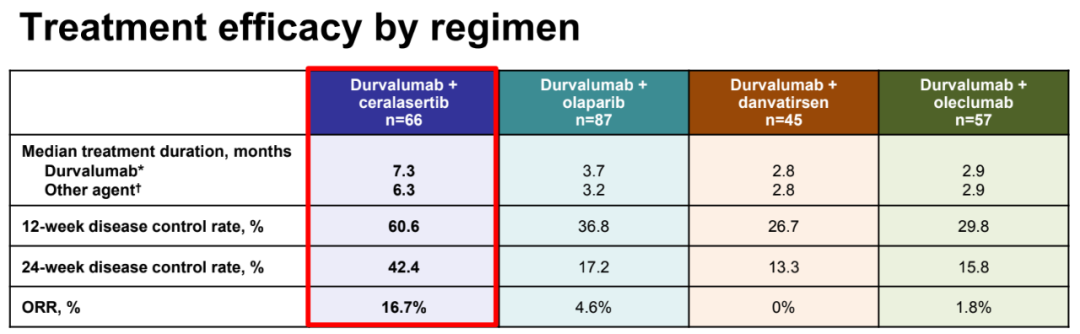
The efficacy data of each treatment queue
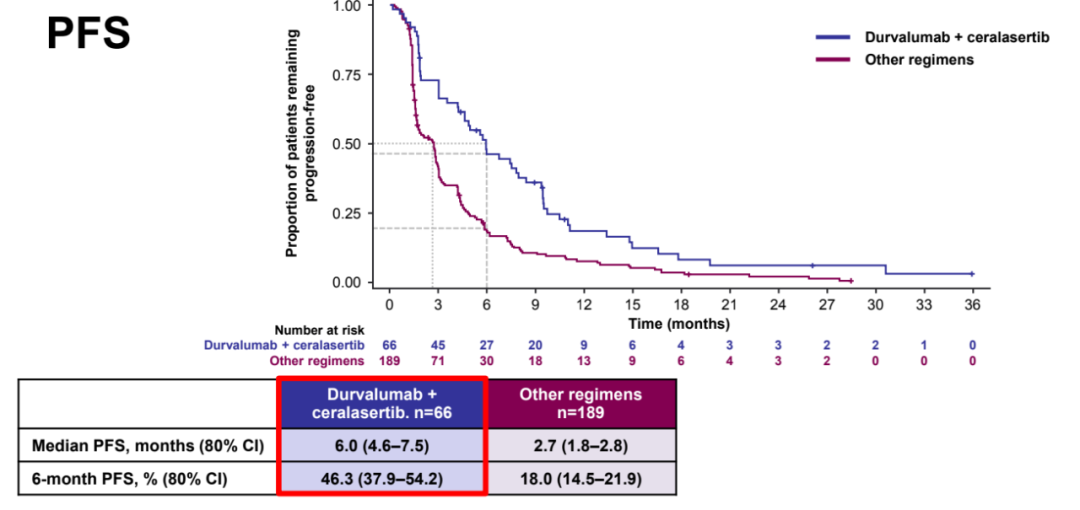
PFS data

OS data
Mid -bit OS is significant in each queue: 22.8 months in the biomarker matching queue (80%CI: 12.6–29.9), and the primary and acquired drug -resistant queues are 11.8 (80%CI, respectively. : 6.6-18.8) and 19.1 (80%CI: 14.1-20.3).
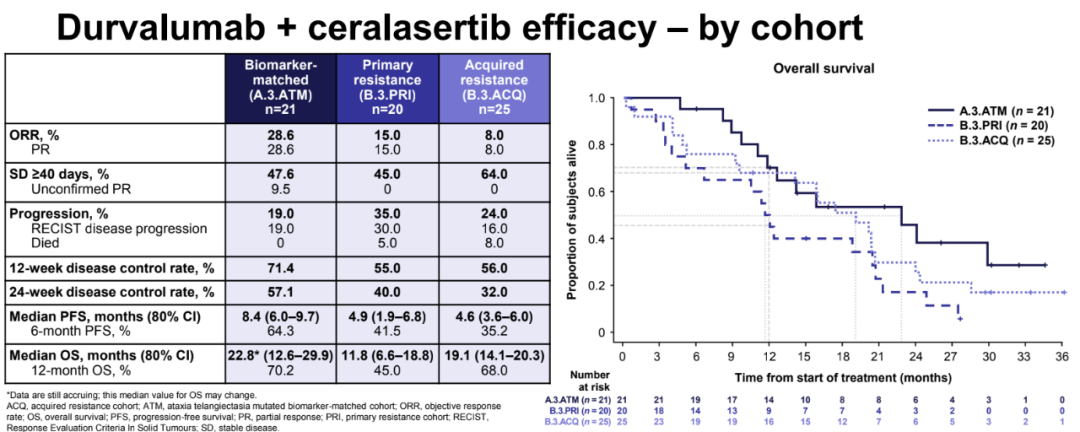
The efficacy data of each treatment queue that matches the biomarkers with a drug -resistant state
Most patients have reportedly report to the treatment related to the treatment of related bad events; compared with other schemes, Diagari Utah combined with Cerasertib or OLCELUMAB level 3 TRAE and the suspension of drugs caused by Tra for is lower. The most common Trams is nausea (the incidence of each treatment group is 42.5%, 2.2%, 51.5%, and 7.0%), anemia (25.3%, 8.9%, 21.2%, 3.5%), fatigue (20.7%, 13.3%, 16.77.7 %, 14.0%), decreased appetite (9.2%, 4.4%, 22.7%, 7.0%), diarrhea (12.6%, 11.1%, 15.2%, 12.3%), and vomiting (20.6%, 4%, 28.8%). Safety data of each treatment queue

The results show that in patients with anti-PD-1/PD-L1 immunotherapy and ≥1 patients with platinum dual drugs failed, Diaapri Mippitive and Cerasertib showed a good efficacy signal with Cerasertib. Tolerable safety.
Pre-Treatment CTDNA Levels Significantly Predicts of OS and PFS in Nadim II TRIAL
N The OS and PFS results of the NADIM II test before the treatment of the CTDNA level before the treatment
(Summary number: MA06.03)
Nawuli Mipida combined with new chemotherapy for the treatment of early non -small cell lung cancer (NSCLC) was approved by FDA in March 2022. Patients with predicting factors can distinguish patients with high or low risk of death in order to adjust subsequent treatment. However, there are currently no biomarkers to identify or screen people who have benefited from immunotherapy combined with the medium and long -term benefits of immunotherapy. Researchers announced the correlation between CTDNA levels and general survival (OS) and no progressive survival (PFS) at this year's conference.
NADIM II is a clinical trial of open label, random, both arm, II phase, and multi -center clinical trial. ECOG PS 0-1, unknown EGFR/ALK changes to the removable IIIA (based on the 7th edition of AJCC) NSCLC patients were randomly assigned to two groups, and the joint treatment group accepted the Neo-Aid Nawulu Mipida (360mg)+paclitaxel (200mg/m2)+card platinum (AUC 5), separate chemotherapy group received paclitaxel (200mg/m2)+card platinum (AUC 5) treatment. It is ± 3 days for 1 cycle for 21 days, 3 cycles, sequential surgical treatment. Patients with pathological assessment confirmed that R0 removed patients in the 3rd to 8 weeks (+7 days) after surgery (480 mg Q4W), which began to receive Nawli Ulitab (480 mg Q4W), for a period of 6 months. Use Trusight Oncology CTDNA Next Generation Sequencing (NGS) to analyze the circulatory tumor DNA (CTDNA) from pre -processing plasma samples.
Test design

The median follow-up time is 21.2 (15.1-25.6) a month. Among the 54 parts of the plasma sample before treatment, 52 parts (91.4%) were detected by the baseline CTDNA, which was significantly related to the tumor size (maximum diameter ≥70mm) (P = 0.006). Before the treatment, the CTDNA level is significantly related to PFS and OS, and has nothing to do with the use threshold. Use <5%mutation equal to the genetic frequency (MAF) threshold (MAF) threshold. In the baseline, compared with patients with high CTDNA levels, PFS and OS of patients with low CTDNA water = flat are significantly improved (for PFS and OSS, HR = 0.19, respectively ; 95%CI: 0.07-0.52; P = 0.013 and HR = 0.13; 95%CI: 0.04-0.45; P = 0.001).
The relationship between CTDNA level and tumor size, PFS and OS

The results show that the baseline CTDNA level can clearly identify patients with high progress and risk of death, and can be used to guide the corresponding follow -up treatment.
Do in Screening-Calf Circumference and Muscle Streangth is Predictive
of Outcomes in LUNG CANCER TREATMENT
Can the calf fence and muscle strength be used as a predictive factors for the treatment of lung cancer?
(Summary number: OA08.03)
Low muscle quality is very common in patients with lung cancer, which can make the treatment rate of toxicity higher, and the physical function, quality of life and mortality is worse. The evaluation method of muscle quality (MM), such as computer fault scanning, is expensive, can be poor, and is not conducive to clinical practice. This study aims to explore the use of simple, low -cost measurement methods [such as the length of the calf (CC) and the grip (HGS)] to measure the mm during diagnosis, and whether it can predict the cancer related prognosis of patients with lung cancer.
This study is a forward-looking and observation clinical study. Data collected the first nutritional consultation from January to December 2019, including nutritional risk screening, height, weight, weight, weight loss of the patient's subjective overall assessment (PG-SGA). (WL), CC, and HGS measured by holding grip meters. The critical value adopted by CC is adjusted by age. The survival curve is generated through Kaplan-Meier to evaluate the correlation between low mm merging and low grip power and 3-year mortality. In this study, a total of 50 patients with lung cancer were admitted to the group, of which 62%were women, 94%were the elderly, 88%were non -small cell lung cancer, 88%were diagnosed with III and stage IV diseases, and 64%were smokers. 62%of patients have WL, and 36%of patients WL is greater than 5%of weight (a standard of measure of evil quality).
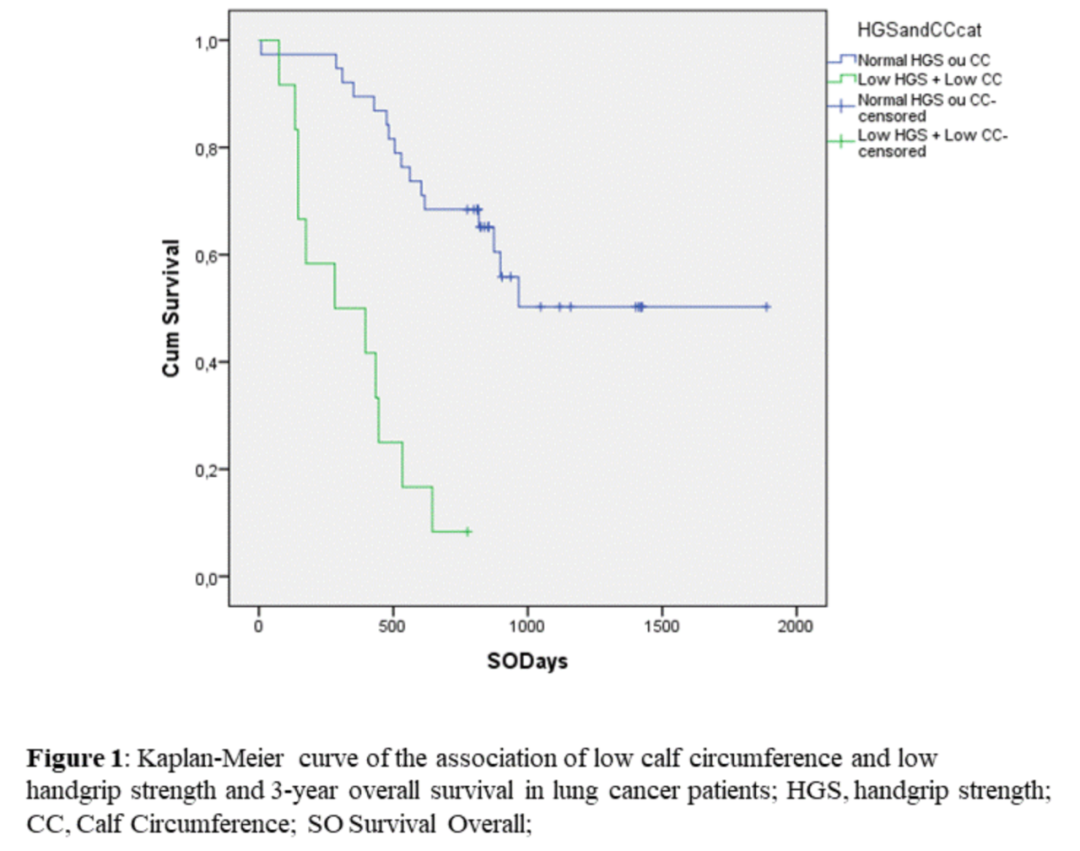
When evaluating the CC of this research object (an easy -to -use MM indicator, which is currently in the adult population), 44%of the patient value is lower than the critical value, indicating that MM is low. In the grip evaluation, 34%of patients have low muscle strength. In the OS assessment, patients with low MM and low muscle strength are significantly lower than patients with higher parameters higher than the definition of critical points (349 days vs 1247 days; for the number of natal P <0.000; Breslow P <0.000; Tarone-Ware P <0.000) Essence Although the sample quantity is limited, the results can be found that the significant differences in the reversal of nutrition and multi -mode intervention can be found.
Patients with lung cancer patients with low legs, low grip and 3-year total survival period of Kaplan-Meier curve
Using an ordinary, simple and durious measurement method, such as WC combined with HGS, the measured low mm is a predictive factors for negative results in lung cancer treatment, which should be paid attention to. As long as early discovery, nutrition and multi -mode intervention can reverse low mm, thereby improving the prognosis of these patients.
Expert Reviews
Immunohistochemical inhibitors (ICIS) have achieved great success in lung cancer treatment, and the exploration of its biomarkers has never stopped. Especially for patients who are resistant to ICIS and immunotherapy, they still lack the ideal logo so far Guide clinical practice. The first study above found that for patients with ICIS drug resistance, through the parallel testing and scheme distribution of multiple logo, it was found that patients with ATM mutations adopted Cauli Mippitarian combined with ATR inhibitors Ceralasertib therapy and obtained it. Very good efficacy and safety. The second study is a sign of the NADIM II test based on new auxiliary treatment of lung cancer. The results found that the baseline CTDNA level can predict patients to benefit the survival of joint treatment. Further achieve accurate immunotherapy.
In addition, the research of prognosis of lung cancer is also the focus of clinicians' attention. The third study of this article uses a simple and easy method. By evaluating muscle quality, it predicts the therapeutic effect of medium and advanced lung cancer, and has achieved better predictive effects. This indicates that it is necessary to further improve the prognosis of lung cancer by improving the nutritional state of patients.
Expert Introduction
Holy Emperor
Doctor of Medicine, Deputy Chief Physician, Cancer Hospital Affiliated to Fudan University

Assistant Director of the Department of Internal Medicine of chest oncology
American Clinical Oncology Society (ASCO) member
Member of the China Anti -Cancer Association Clinical Oncology Chemotherapy Committee
Secretary of COE of the China Pharmaceutical Education Association of the Cycies
Member of the China Clinical Oncology Society (CSCO)
Member of Shanghai Anti -Cancer Association
Standing Committee Member of the Shanghai Anti -Cancer Association CRPC Youth Committee
The internal medicine treatment and research of chest tumors such as lung cancer and esophageal cancer, in depth research in the fields of new target identification, molecular logo research, tumor palliative treatment, etc. have published more than ten SCI papers and presided over 2 scientific research funds.
references:
[1] Mark M.awad, et al.OA15.05-HUDSON: An Open-Label, Multi-Drug, Biomarker-Direct Phase 2 Study in NSCLC Patients Who-pd- (L) 1 Therapy.2022W2222222222222222222222222222222222222222222222222222wclc. OA15.05.
[2] Roberto Serna, et al.MA06.03-PRE-TREATMENT CTDNA Levels Significantly Predicts of OS and PFS in Nadim II TRIAL.202222CLC.MA06.03.
[3] Gisele Fraga Moreira, et al.OA08.03-DO in Screening-Calf Circumference and Muscle Streangth is Predictive of Outcom Cancer Treatment.2022222Clc.08.03.
The first release of this article: the medical world tumor channel
Author of this article: lily
Editor in charge: Sweet
- END -
Can "magic weight loss needle" really return your devil figure?

Recently, a magical weight loss needle is very popular in the circle of friends. S...
Monkey Acne diagnosis and treatment guide (2022 edition)
Monkey acne diagnosis and treatment guide(2022 edition)Monkey acne is a viral dise...