The new "Medicine King" emerged, and the price of 100 billion products reduced the price by 55%to face, and the domestic enterprise was one step away.
Author:Kenji Bureau Time:2022.06.18
The annual conference of the American Clinical Oncology Society (ASCO), which is held in early June, is the stage for the world's anticancer drug competition.
At the ASCO Annual Conference 10 years ago, the scientists of Johns Hopkins University took out the drugs numbered BMS-936558, which was a PD-1 that was later represented. Since then, the industry has been looking forward to the second "PD-1" on ASCO, which once again leads the development direction of global pharmaceutical companies.
At the ASCO Annual Conference in 2022, the industry saw hope.
This time, ADC drug DS-8201 data for breast cancer is stunning: For patients with low levels of HER2, HR positive, non-removal or metastatic breast cancer, this drug can reduce the risk of cancer progress by 49%, The risk of death is reduced by 36%.
Compared with the control group, the DS-8201 extended the patient's no progressive survival period from 5.4 months to 10.1 months; the total survival period was increased from 17.5 months to 23.9 months.
Ordinary people may not be able to understand the significance of these numbers, but for breast cancer therapy experts, this ADC drug can be said to achieve "unprecedented effects." At the same time, this medicine will also rewrite the classification and treatment of current breast cancer.
Breast cancer has long exceeded lung cancer and has become the world's largest cancer species. Over the past 20 years, Roche's Twozumab ("Herceptin") has dominated the world and is one of the most popular breast cancer therapy drugs. The emergence of DS-8201 may lead to re-sorting the world's best-selling anticancer drugs.
This time, will domestic companies still be at no way?
"Kill" Qi has arrived
DS-8201 was menacing, and it was Roche's Tusko Mometeriopato.
In September 1998, Roche's Telkomo Mipido was approved to be listed in the United States. This is the world's first HER2 target monoclonal anti -resistance, which is directly treated with breast cancer. The most antiprolee of Tushuzhu's anti -header has brought the return of real gold and silver to Roche.
For more than 20 years, Tusko Mutterum has become the best choice for breast cancer treatment. It has long occupied the global drug sales TOP10 list, contributing Roche's revenue of over 100 billion US dollars.
In 2014, the protected patent of Tuskomo Metrics expired, and many generic drug companies poured into this treasure track. Roche is also constantly building a high city wall and developed the ADC version of the Tuskomo monoclonal anti -anti -resistance: Enmeiustezumab.
ADC drugs are composed of three parts: antibodies, connectors and toxins, antibodies and toxins can be used together to kill cancer cells more thoroughly. The ADC version of the Twozumab, the effect is a level higher than that of the single -used monopoly.
In terms of the treatment of breast cancer, the barriers are high enough, but the DS-8201 appears.
DS-8201 is an ADC product that is also used as antibodies with Tuskomo, but its curative effect is significantly better than Enmengustezumab. In September 2021, at the General Assembly of the European Cancer Science Society, DS-8201 announced the first clinical trial data of patients with Enmegangzumouzab the treatment HER2 positive, non-removed or metastatic breast cancer patients: DS-8201 ratio than The risk of the progress or death of the monoclonal resistance of Enmei Qucin decreased by 72%. At the same time, the non-progressive survival period of the DS-8201 was 25.1 months, and the Enmei Qukopo monocidal was only 7.2 months.
Since then, DS-8201 is like a shadow, shrouded in Roche's "Toppuzhu Castle".
In November 2021, the National Comprehensive Cancer Network NCCN updated the "Guide to the Clinical Practice of Breast Cancer (the first edition of 2022)". In the second-line solution for Her2-positive, recurrence, irregularity, or phase IV disease, DS-8201 replaced Enmeiustezumab and became the first choice plan. Roche ADC was replaced to category 2A or other solutions middle.
The US update diagnosis and treatment guide has a reference significance for the treatment of global breast cancer. In March 2022, DS-8201's listing of listing in China was accepted; April, DS-8201 treatment had previously received one or more anti-HER2 drug treatment irreplaceable or metastatic HER2 positive breast cancer adult patients received China Certification of breakthrough therapy of the State Drug Administration.
Roche began to take the initiative to reduce prices and strive to seize the larger market before landing in China.
今年上半年,罗氏对恩美曲妥珠单抗的价格进行调整,规格100mg的价格由近2万元降至不足8500元;160mg的价格由2.7万余元降至1.3万余元,降幅超过55%.
Heavy bet
DS-8201 has the strength to rewrite the industry pattern, and there must be a lot of competition around it.
Like PD-1, DS-8201 originated from Japan. Around 2007, Japan's First Pharmaceutical Co., Ltd. and the Three Communist Co., Ltd. seemed to be consolidated. The first pharmaceutical accumulated deep accumulation of anti -cancer drugs, and the three Communist Pharmaceuticals had special strengths in the field of humanized antibodies. After the merger, ADC drugs were quickly identified as the key projects of the newly established "first and third communist".
At that time, ADC drugs were also very cutting -edge technologies. The first three tried attempts in the field of ADC were set in the field of breast cancer for HER2 genes. Similar to PD-1, HER2 gene abnormality is manifested in bladder cancer, cervical cancer, bile duct cancer, colorectal cancer and other cancers are manifested, and it is the most common in breast cancer. Moreover, the Tushuzhu Mipoic treatment HER2 -positive breast cancer that targeted HER2 has been verified. This is the main reason why the HER2 target ADC drugs are studied in the first three and three points. At the end of August 2017, the DS-8201 developed by the first and third was determined by the breakthrough therapy issued by the US FDA. Soon, Astrakang noticed this product.
Astrakang also wanted to do ADC. At that time, the ADC therapeutics, the main ADC product, was invested. However, the related projects of this company were still in the early clinical stage and did not get the product. In March 2019, Astraika obtained the commercial rights and interests of DS-8201 outside Japan for $ 6.9 billion. In December of that year, DS-8201 was approved to be listed in the United States.
Take the DS-8201 to become an important step for Astraikon's joining the ADC track, and also allowed Astraon to challenge Roche Roche, Roche, challenging Breast cancer treatment.
Astraon is currently deepening cooperation with the ADC track with the first and third. In July 2020, another ADC product DS-1062, which targeted Trop2, was also won by Astraikon.
Stringer
During the monoclonal era, Chinese companies could hardly make representative varieties. However, when ADC rose, domestic companies quickly kept up with this trend: as of the beginning of June, more than 200 ADC projects in the clinical stage of the clinical stage, of which nearly 60 were in China.
At present, 9 ADC drugs around the world have been approved for listing. There are three models in the Chinese market, namely Roche's Enmeius Midurate, Vidici Mipido of Rongchang creatures, and Genting's new Yao Goshzukeke.
Picture source: Southwest Securities & Public Information Organization
However, in the treatment of HER2, there are currently only three products in the world that only have Enmei Qukopo Ming, DS-8201 and Vidici Mipido. DS-8201 has not been approved in China, and the product indications of Rongchang Bio and Roche have staggered.
This kind of life will not last for a long time. As mentioned earlier: March this year, the domestic listing application in DS-8201 was accepted; in April, it was certified by breakthrough therapy. DS-8201 may come at any time.
Among the domestic research ADC, the proportion of HER2 is the most targeted. These varieties are mostly borrowed from Enmengustezab, and DS-8201 may constitute a decrease in dimensions of these products.
Picture source: Southwest Securities & Pharmaceutical Notes & Public Information Sorting
Although the DS-8201 is outstanding in the treatment of breast cancer, Astraikon and the first and third Communists do not want to limit it to the field of breast cancer, but hope it can play a role in various cancer species.
From the perspective of the drug clinical trial registration and information publicity platform of the State Drug Administration, DS-8201 has been carried out in Phase III in the treatment of at least three indications such as breast cancer and gastric cancer. Once approved, it is likely to be fast quickly. Play multiple indications.
Take non -small cell lung cancer as an example. DS-8201 has announced phase II/III clinical data for patients with complex refractory non-small cell lung cancer. According to some of the relevant clinical data released earlier, among patients with metastatic non -small cell lung cancer caused by the treatment of HER2 excessive expression, the total relief rate reached 24.5%, the disease control rate reached 69.4%, and the mid -level no progressive survival period reached 5.4 months. Essence The data is deemed to have clinical significance.
As early as 2019, the first three Communist Party stated at the R & D day activities that it will jointly conduct 43 clinical trials with Astraikon to cover patients with symptoms such as lung cancer, gastric cancer, colorectal cancer, and "unlimited cancer species". Try to use DS-8201 to change the treatment of HER2 positive tumor.
DS-8201 is very likely to become a new generation of "magic medicine" and change the commercial pattern of physical tumors.
This time, domestic companies can no longer be regarded as "we can't reach". After all, the development of related ADC drugs is not too late. But looking at the taste of the item is equally uncomfortable. Fortunately, the gap is not far away, and it is unknown whether it can be caught up.
Writing | Nicotinamide
Edit | Jiang Yun Jia Ting
Illustration | Visual China
Operation | Valley
#Breast cancer#
- END -
Will cataract surgery be infected in summer?Don't be pitted by these old ideas, listen to what experts say
Old man, you are suffering from cataract, your vision is already poor, you can't d...
Another License Out!East China Pharmaceutical Liculucan peptide is as soon as possible
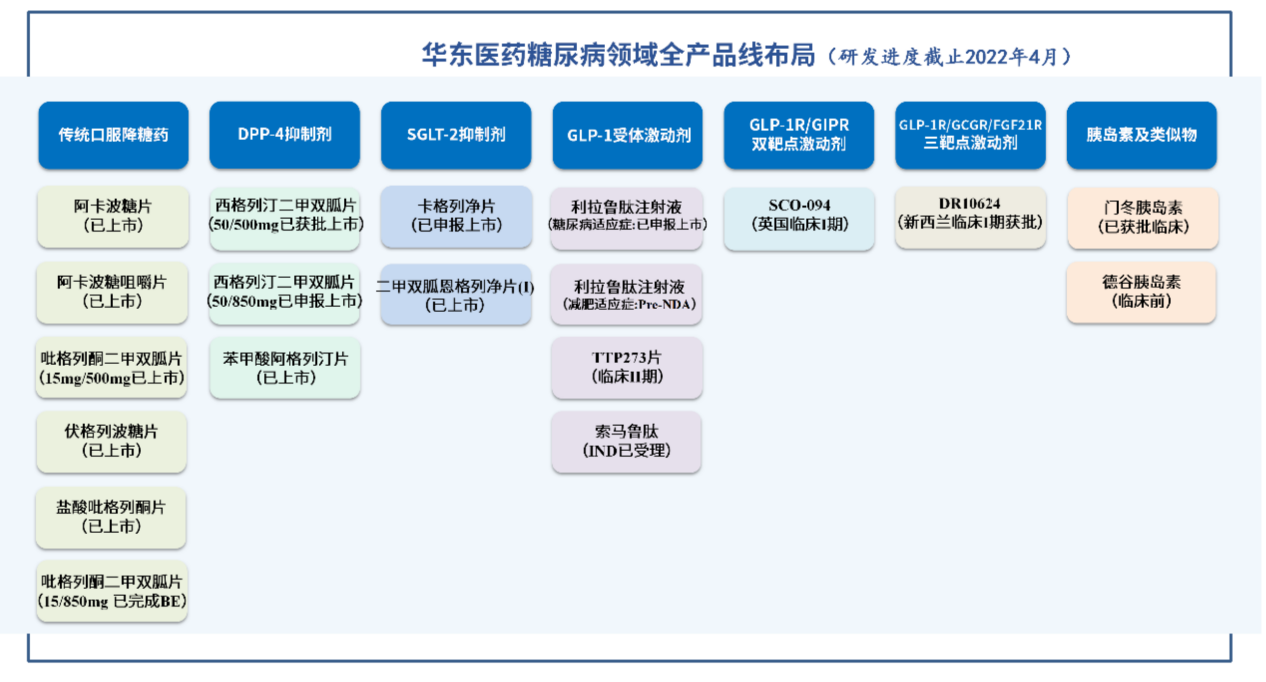
On June 23, East China Pharmaceutical (SZ.000963) announced that it would reach a ...