Is small cell lung cancer treatment tricky?4 Studies indicate direction | 2022WCLC
Author:Cancer Channel of the Medical Time:2022.08.24
*For medical professionals for reading reference

SCLC emerging treatment means frequently, and the medical community takes you to watch the latest progress in SCLC treatment in WCLC!
In the past, due to the lack of corresponding driving genes of small cell lung cancer (SCLC), it was lacking in treatment. With the advent of the era of immunotherapy and the deepening of accurate treatment concepts, SCLC's treatment has also ushered in revolutionary changes. At the World Lung Cancer Conference (WCLC) in 2022, the research results in the field of SCLC treatment were announced, let us see it.
Phase 2 Study Analysis of Talazoparib (TALA) Plus Temozolomide (TMZ) for Extensive-Stage Small Cell LUNG Cancer (ES-SCLC)
剂PARP inhibitors combined with low doses of olizerrazib can benefit patients with patients with small cell lung cancer
Summary number: OA12.03
TALA shows cytotoxicity by inhibiting the polyetric ADP nucleose polymerase (PARP) protein 1 and 2 and the PARP on DNA. In the model of small cell lung carcinoma (SCLC), studies have confirmed that the combined TALA combined with TALA can enhance the anti -tumor effect (Wainberg AACR 2016). Therefore, Professor J.Goldman from the United States believes that TALA is combined with omolizer as a second -line treatment to improve the ending of SCLC patients.
2022WCLC Abstract Screenshot
The study is a stage II open label and one -arm study. It aims to detect the recurrence / difficult period SCLC patients who fail to treat in the first line of platinum. A total of 31 patients with SCLC patients were entered in a total of 31 patients, of which 28 patients had the curative effect of patients. All patients who were admitted for 28 days were one cycle and accepted TALA [daily orally 0.75mg (creatinine clearance <60 60 60 Patients of ml/min receive 0.5 mg) and treat them for oral omolizer (3.75mg/m2) at the first to 5th day of each cycle. The research results show:
Patients with 11/28 (39.3%) have reached partial relief (PR);
In the sensitive sub -groups of platinum difficulty, platinum resistant, platinum, and platinum, the objective relief rate (ORR) is similar, respectively, 3/6, 4/9, and 4/13;
The median time for treatment is 1.8 months, the duration of the duration is 4.3 months, the survival time of no disease is 4.3 months, and the total survival time is 11.9 months (Figure 1);
Adverse reactions are controllable. The adverse reactions at level 3 are platelet reduction (61.3%), anemia (54.8%), a decrease in neutral granulocytes (41.9%), and atypical pneumonia (3.2%). These adverse reactions are maintaining the dose dose Or reduce the treatment and blood transfusion and infusion growth factor in a good state;
Cell free DNA and tissue analysis showed that no DNA damage response (DDR) mutations (DDR) mutations were found among the subjects. treat.
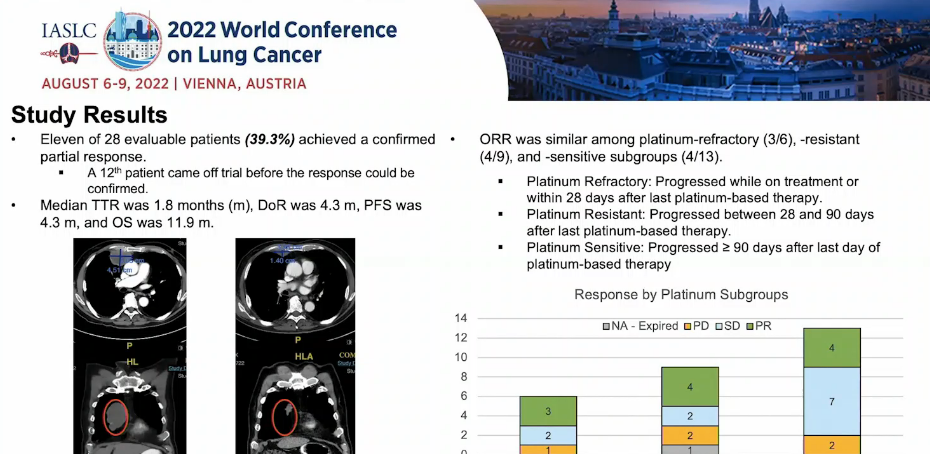
Figure 1: The results of the efficacy in the study
In short, the study exceeds the target reaction rate. This is the second research on the effect of PARP inhibitors and low -dose olizer on SCLC. In the future, phase III research will prove the advantages and disadvantages of this treatment model and the current approval treatment model.
Effical of Nivolumab and Temozolomide in Extensive Stage Small Cell LUNG CARL AFTER Chemo-Immunotherapy: A Phase 2 TRIAL
单 Nawuli Mipida combined with omoloraz is the potential for the treatment of second and third -line treatment of SCLC in a wide range of SCLC second and third -line treatment
Summary number: OA12.04
After the use of chemotherapy combined with chemotherapy combined immunotherapy (CIT), the extensive period of SCLC occurred, and subsequent treatment options are often very limited. Temoraz has anti -tumor activity in the treatment of SCLC in a wide range, and has confirmed immune regulation in advanced tumors, but lacks data after CIT treatment. Therefore, a phase II study reported at the WCLC conference aims to study the extensive period SCLC patients that progress after using CIT treatment, and use NCT03728361 as a second or third -line treatment with Naweli Ulitab.
2022WCLC Abstract Screenshot
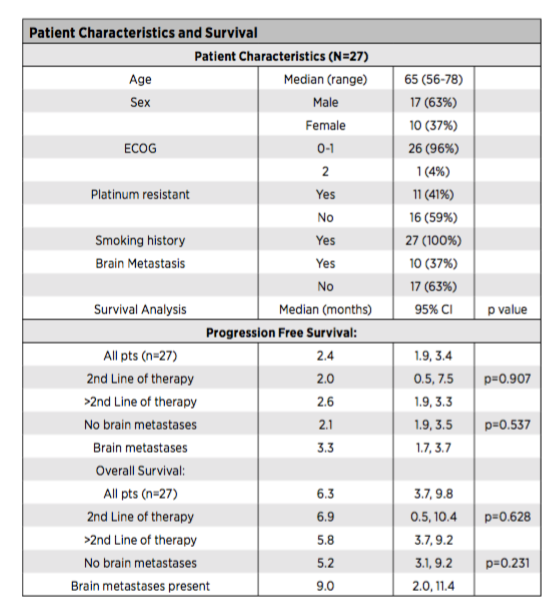
NCT03728361 is a phase II clinical study of non -random, multi -center, single center, open label. The patients incorporated are the extensive period of SCLC and patients with a separate reporting nerve endocrine tumor reported by CIT for treatment after CIT treatment. The treatment is 28 days as a cycle, of which 5 days to Nawuli Mipidic (480mg) intravenous infusion and deorzolizer 150mg /m2. A total of 27 patients were included in the study, of which 11 (41%) were platinum resistant patients, and 10 (37%) were patients with brain metastases (Table 1). 25 patients had progressed after using CIT treatment, and the main research final analysis was conducted. The results showed that among the patients who used to develop CIT treatment in the past, 7/25 cases (28%, 95%CI 12%-49%) Patients used Nawuli Ulitaba to combine the treatment response, and 8/27 cases (30%) in the overall population reached the preset efficiency standard;
The median and medium OS of the overall crowd are 2.4 months (95%CI 1.9-3.4) and 6.3 months (95%CI 3.7-9.8) (Table 1). OS has nothing to do with the number of treatment lines and central metastasis. It
The median OS time for patients with brain metastases is 9 months (95%CI 2.0-11.4) (Table 1);
Toxicity is similar to CIT treatment strategies.
Table 1: Patient characteristics (above), patient PFS situation (below)
Figure 2: The changes in the lesions of patients since the baseline
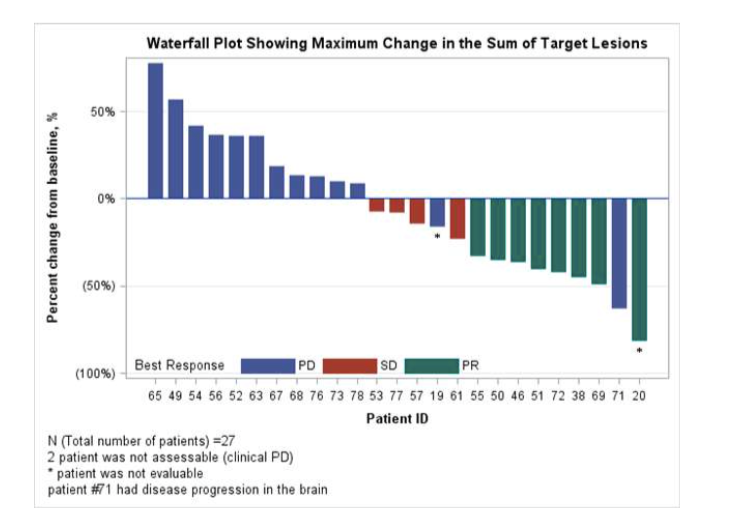
In short, for patients with a wide range of SCLC in the advancement of the first -line CIT treatment, Nawli Ulitaba combined with molezide as second and third -line treatment and the treatment potential in the treatment of SCLC patients with brain metastases.
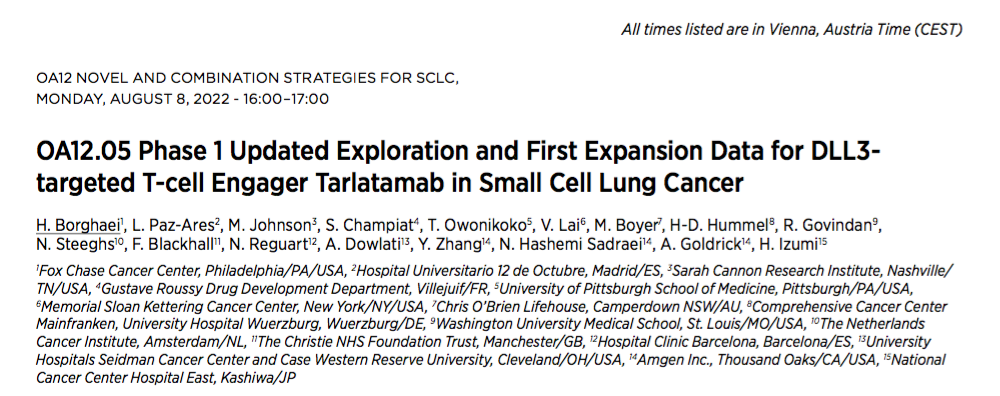
Phase 1 Updated Exploration and First Expansion Data for DLL3-targeted T-cell Engager Tarlatamab in Small Cell Lung Cancer
治 TARLATAMB shows the promising effect in the treatment of SCLC patients who have previously treated SCLC
Summary number: OA12.05
There are triangular ligands (DLL3) in most SCLC patients. Tarlatamab (AMG757) is a half -life extended bic cell connector (HLE BITE®), which can be combined with DLL3 and CD3, causing T cell media to dissolve tumor. Tarlatamab treats the mid -term I dose exploration study of SCLC (NCT03319940), which shows preliminary efficacy evidence and has acceptable security. The researchers first reported the safety, relief rate and survival data of the joint dose exploration and extended queue.
2022WCLC Abstract Screenshot
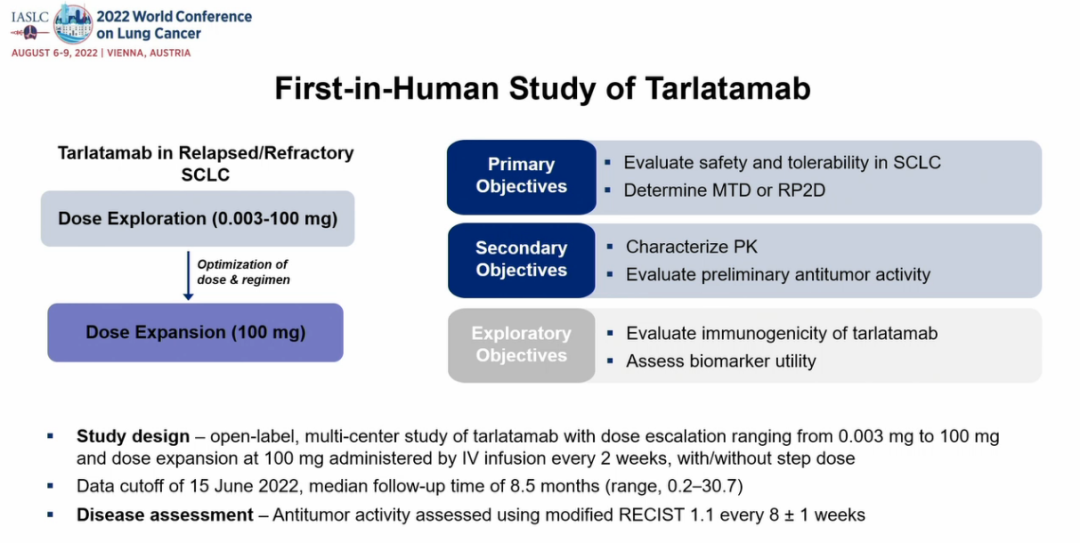
The Dellphi-300 Study is the first human test of Tarlatamab (Figure 3). The dosage exploration range is 0.003-100.0 mg. The patients incorporated were previously received SCLC patients with ≥1 platinum-containing treatment schemes. Among the dosage exploration and extension queue, 102 patients accepted the progress/recurrence of the disease after the ≥1 line -containing chemotherapy, and accepted the ≥1 dose of Tarlatamab in the dose exploration and extension queue. The median follow -up time was 8.5 months. The
Among the patients with a medium early treatment of SCLC, the ORR of Tarlatamab reached 23%(95%CI 15.8%-33.7%), and the disease control rate (DCR) was 55%;
The duration of the median relief (DOR) is 13 months, and the median OS is 13.2 months (95%CI 8.8-not achieved);
In terms of safety, 2%of patients have any level of treatment related to adverse reactions (Traes), of which 31%of ≥ 3; 1 patient (1.0%) of level 3 cytokines related to treatment (CRS), CRS, CRS), CRS, 7 patients (7%) had a treatment -related 3 -level nervous system incident;
Figure 3: Research Design
In short, for patients with severe treatment in the past, Tarlatamab shows the safety of treatment prospects and controllable. Compared with other treatment methods currently recur's SCLC, PFS and OS are comparable. Give this result, Tarlatamab is undergoing phase II research for SCLC third and above.
OA12.06 First-Line PEMBRolizumab or Placebo Combined with Etoposide and Platinum for ES-SCLC: Keynote-604 Long-Term Follow-Up Results
The long-term follow-up results of eKeynote-604 show that Paborizumab + EP can bring benefits to patients with a wide range of SCLC
Phase III Study Keynote-604 prompts. Compared with the placebo combination of the placebo, the first-line treatment of Paborizumab combined with EP scheme as a wide range of SCLC can significantly improve PFS (HR = 0.75, 95%CI 0.61-0.91 ; P = 0.0023), at the same time, the patient's overall survival (OS) also showed a tendency to benefit, but did not reach a significant threshold. After about 3.5 years of follow -up for patients who completed the 35 -cycle Paborzab's treatment, Professor C.M.RUDIN from the United States gave the latest analysis results here. 2022WCLC Abstract Screenshot
As of September 21, 2021, a total of 453 patients in the treatment of the treatment (ITT) were included in 453 patients [Pabberzumab+EP (228 cases); placebo+EP (225 cases)]. 54.8%of patients in Paborzab+EP group and 66.2%of patients with placebo+EP group received follow -up treatment. Among them, the proportion of patients who received follow -up immune examination point inhibitors were 11.2%and 22.1%, respectively. Analysis shows:

OS analysis shows that Paborzab's Mipide+EP improves the survival benefits of patients. The median OS of the two groups of patients is 10.8 months (95%CI 9.2-12.9) and 9.7 months (95%CI 9.2-12.99.9 ), HR = 0.76 (95%CI 0.63-0.93), the 3-year OS rate was 15.5%and 5.9%, respectively (Figure 4);
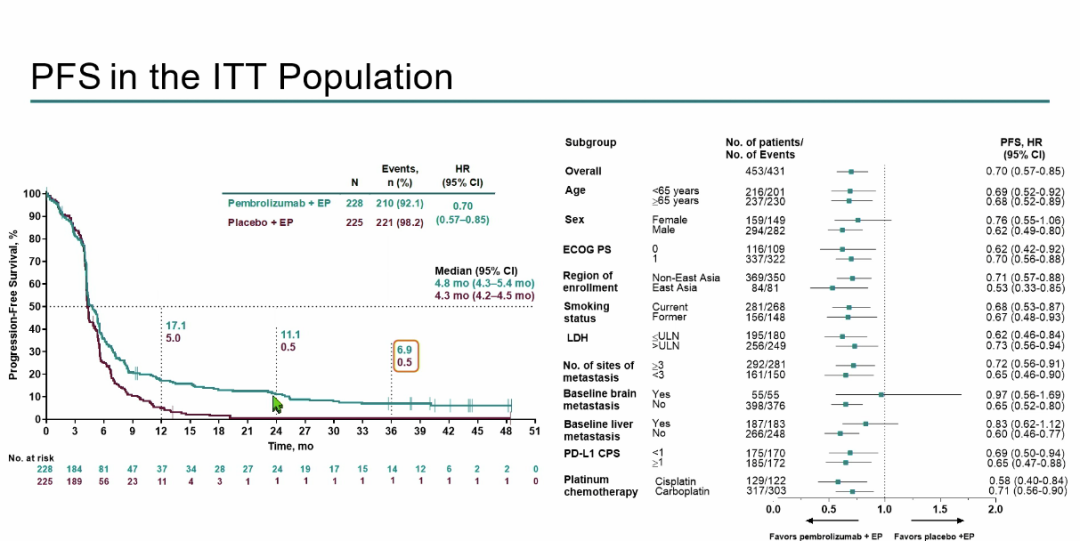
PFS analysis shows that the median PFS of the Paborzab+EP group is 4.8 months (95%CI 4.3-5.4), and the median PFS of the placebo+EP group is 4.3 months (95%CI 4.2-4.5) The 3 -year PFS rates of the two groups are 6.9%and 0.5%respectively (Figure 5);
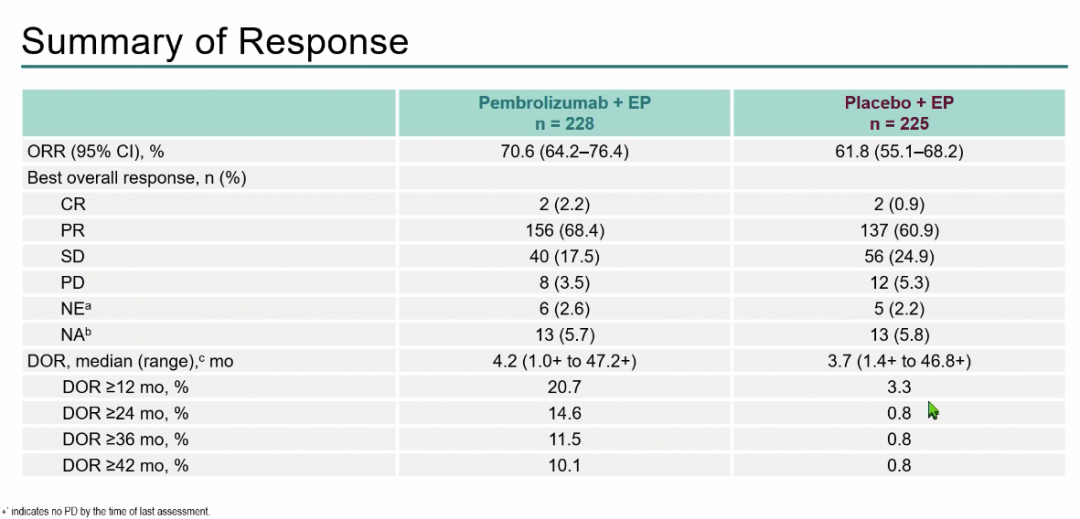
According to the analysis of the patient's disease relief, the ORR of the Paborizumab+EP group is 70.6%, and the median Dor is 4.2 months; Image 6);
Safety analysis shows that 78.9%and 77.1%of patients in Pabberzumab+EP group and placebo+EP group cause any causes of 3 to 5 adverse events caused by any causes. The incidence of two groups of immune -related adverse events was 27.4%and 12.1%, respectively, of which the incidence of non -performing related adverse events of level 3 to 5 was 8.1%and 1.3%, respectively;
A total of 18 patients completed 35 cycles of Paborzumab. The ORR of 18 patients was 100%(95%CI 81.5%-100%). Beginning with the 35-cycle Paborzab (about 2 years), the patient's 2-year OS rate was 72.2%(95%CI 39.5%-89.2%).
Figure 4: ITT crowd OS results
Figure 5: ITT crowd PFS results
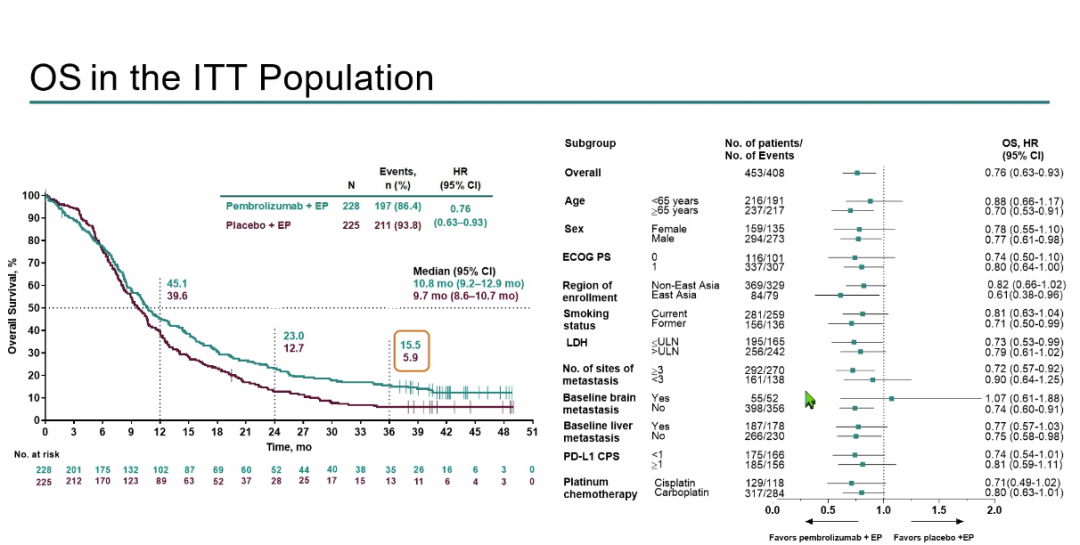
Figure 6: Treatment reaction results
In short, for patients with ES-SCLC who have not been treated, compared with placebo+EP, Paborzab's Mipide+EP still shows better survival benefits and management security in long-term follow-up. Patablyzab+EP group patients in the 3 -year OS rate can reach 15.5%, while the control group is 5.9%. Patients who complete all the treatment cycles can get better survival benefits. Based on the above data, the researchers believe that the application of Paborizumab joint strategy should continue to be applied in patients with ES-SCLC.
references:
[1] OA12.03.2022 WCLC.
[2] OA12.04.2022 WCLC.
[3] OA12.05.2022 WCLC.
[4] OA12.06.2022 WCLC.
The first release of this article: the medical world tumor channel
Author: Tumor Baby
Editor in charge: Sweet
- END -
Anti -"epidemic" has our teacher first

Faced with the sudden epidemic, 67 teachers in the Lanmian Factory community in Xi...
The 6 "cancer signals" common on the B -ultrasound report, please see if you have any
*For medical professionals for reading referenceQuickly repost to the person in ne...