MET, NTRK, EGFR EXON20INS targeted therapy latest progress 丨 2022 WCLC
Author:Cancer Channel of the Medical Time:2022.08.17
*For medical professionals for reading reference

Research Progress Express
From August 6th to 9th, 2022, the World Lung Cancer Conference (WCLC) was held in Vienna, Austria, and brought many progress to the diagnosis and treatment of lung cancer in Vienna, Austria. At this meeting, there are also a number of research progress on rare targets. Let's follow this article for us to understand!

Scan the two -dimensional code above, get WCLC cutting -edge information and expert views
Savannah Research: Savacinib and Oshitinib jointly treated high MET to express lung cancer, Orr reached 49%
Savannah research is a random, one -arm, and global phase II clinical trial in progress. It aims to evaluate the EGFR mutations that have been treated with the progress of diseases after the treatment of Oshitinib for treatment in the treatment of Saivininib and Oshitinib therapy. MET amplification or expressed local advanced or metastatic non -small cell lung cancer patients. This is the world's first phase II clinical study for MET amplification and/or MET for the three generations of EGFR-TKI Oshitininib. (Summary number: EP08.02-140)
Patients were treated with Oshitinib (80mg QD) combined with Saivininib (300/600mg QD or 300mg BID). The main end point is objective relief rate (ORR). Eligible MET abnormalities include MET amplification detected by fluorescence in situ (FISH) [MET copy number ≥ 5 and/or MET: CEP signal ratio ≥ 2 (FISH5+)] or through the immunohistochemical staining method (IHC) The detected MET expression [≥50%of tumor cells 3+ (IHC50+)].
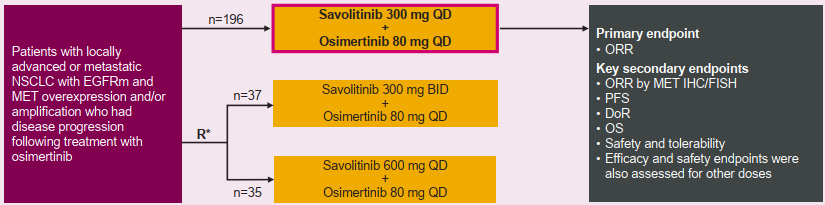
Figure 1: Research Design
This study initially analyzed 196 patient data for the treatment of 300 mg QD combined with Oshitinib 80 mg QD. As of August 27, 2021, the efficacy of 193 patients could be evaluated, of which 108 (56%) were IHC90+and/or FISH10+.
The results of the study show that for patients with higher METs higher than expression, the combination of Savidinib combined with Oshitinib two targets is more significant. The ORR of IHC90+and/or FISH10+Asian group is 49%, and there is no progressive survival (MPFS) in the median (MPFS). The value is higher than the IHC50+and/or FISH5+group (32%, MPFS is 5.3 months). And non -IHC90+and/or FISH10+sub -group (ORR is 9%and MPFS is 2.8 months). The tumor relief of the IHC90+and/or FISH10+sub -group is also more durable. The mid -level relief duration (MDOR) is 9.3 months (the median follow -up time is 13 months, and the DOR is immature). Among all 196 patients, the median treatment time for Savininib and Oshitinib was 4.8 months and 4.9 months, respectively. The safety of each drug and combined treatment is consistent with previous research, and no new security issues are found.
Table 1: Main efficacy data
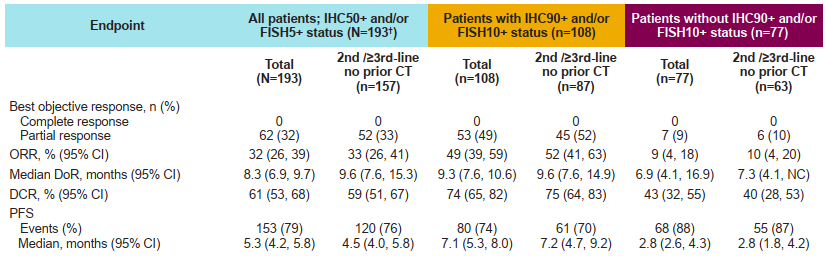
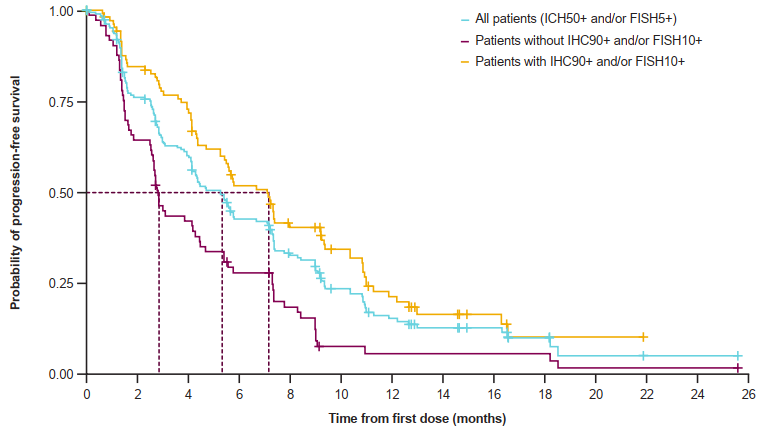
Figure 2: PFS data
Larotinib the treatment of long -term follow -up data update of TRK fusion lung cancer: ORR is 83%, OS is 40.7 months
Larotinib is a high -selective TRK inhibitor with a central nervous system (CNS) activity. As of July 2020 (NCT02576431, NCT02122913), Larotinib was evaluated by the Independent Examination Committee (IRC) evaluation of the Independent Centers (IRC) in 15 cases of TRK (queue 1) in 15 cases of TRK. To. The meeting updated the long -term follow -up data and expansion data of these patients. (Summary number: EP08.02-148)
As of July 2021, a total of 26 patients with NTRK genes fused lung cancer patients (queue 2), of which patients with NTRK1 and NTRK3 gene fusion were 21 cases (81%) and 5 cases (19%). 2 medium -ranked systemic treatment schemes.
A queue 2 patients received Roninib for treatment from 2.1 months to 52.7+ months. Among the 23 patients who can evaluate the effect of queue 2, the ORR evaluated by IRC was 83%; MDOR and MPFS did not reach, and the median total survival (MOS) was 40.7 months; the 24 -month DOR rate, PFS rate and OS The rates are 72%, 67%, and 72%, respectively. Among them, the ORR of 10 patients with the baseline CNS metastasis was 80%, MDOR, MPFS, and MOS were 9.5 months, 9.9 months and 19.4 months.
Table 2: Main efficacy data
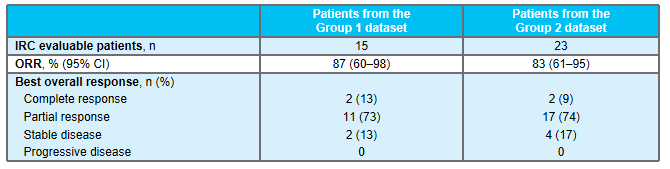
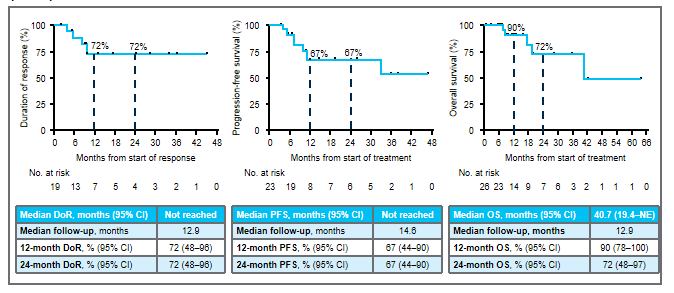
Figure 3: Main survival data
The 15 patients with lung cancer 1 after 24 months were followed by MDOR. After 35.8 months follow -up, MPFS and MOS were 33 months and 40.7 months, respectively.
In terms of security, there is no new security signal in queue 1. The treatment related adverse events (Traes) in the study in the study is mostly 1-2, and no patient is interrupted by Traes.
Wu-kong1/2/6 Studies: Schwotinib backline to treat EGFR EXON20INS mutations NSCLC, Orr reaches 52.4%Schwotinib is a orally, high-selective EGFR tyrosine for multiple EGFR mutations subcontrays Kitase inhibitor. The data reported at this WCLC conference comes from the summary analysis of the three multi-centered clinical research (WU-KOong1, Wu-Kong2, and Wu-Kong6) of Schwotinib at this WCLC conference. (Summary number: EP08.02-029)
Figure 4: Research Design
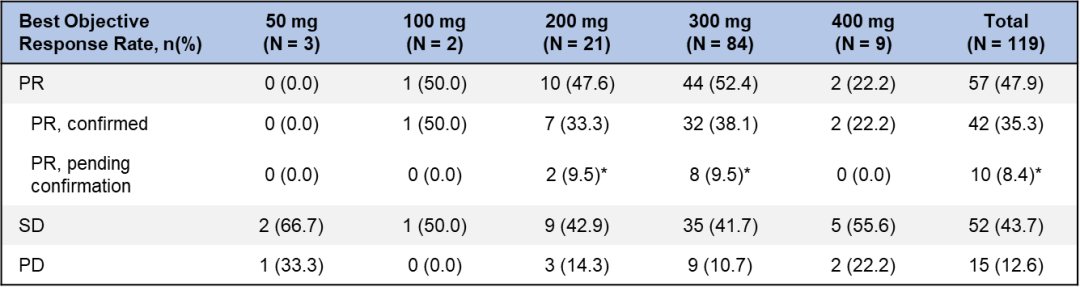
As of April 30, 2022, a total of 119 patients with chemotherapy failed and EGFR EXON20INS mutated late NSCLC patients were included in the efficacy analysis set.
The results of the study showed that 84 patients who were treated with Schwotinini 300mg QD were as high as 52.4%; Schwotinini also showed good anti -tumor activity with a brain metastases against the baseline, and ORR reached 44%.
Table 3: Main efficacy data
There are about 30 types of subtypes in EGFR EXON20INS mutations. Regardless of the position of inserting mutations, most subtypes can benefit from the treatment of Schwotinib: 82 patients inserted mutations occur at the Near-Loop (Near-Loop ), Orr reached 52.4%, the disease control rate (DCR) reached 89%; 29 patients inserted the mutation position at the far-loop, Orr reached 41.4%, and DCR reached 86.2%.
references:
[1] zhou q, et al.Sanovo: a Phase 3 Study of Savolitinib or Placebo in Combining with Osimertinib in Patients with EGFR-Mutant and Met Overessed NSClc.
[2] v. Moreno, et al. Extended follow-up of efficacy and safety of larotrectinib in patients with trk fusion Lung Cancer. WCLC 2022.08.02-148.
[3] J.C-H. Yang, et al.Sunvozrtinib in NSCLC Patients with EgFR EXON20 Insertion Mutations: Effect of Prior Treatment. WCLC 2022.EP08.02-02999.
not
First of this article: Rare target ecosystem in the medical community
Organize this article: Re
Editor in charge: Sweet
- END -
A hospital for three days and three strokes, hot stroke?7 keywords are safe!
On July 22, according to the public account of the Affiliated Hospital of Hangzhou Normal University, the Stroke Center of the hospital recently accepted three patients with three consecutive cerebral
New "11+41"
At 0-24 on June 17, 31 provinces (autonomous regions, municipalities) and Xinjiang Production and Construction Corps reported 35 newly confirmed cases. Among them, there were 24 overseas input cases (