This test is cold in the test of Mer Shadong.
Author:Zhongxin Jingwei Time:2022.08.09
Zhongxin Jingwei, August 9th (Wang Yuling) Recently, Merhado announced its PD-1 inhibitor Keytruda (Paborizumab, often referred to as K medicine). The clinical trials of the III fail.
The clinical trial belongs to the joint medication and is called the "Cola combination". Zhongxin Jingwei noticed that many pharmaceutical companies such as Junshi Biological and Kangfang Bio, China, also opened similar clinical trials. What does the failure of the above clinical trials mean that these pharmaceutical companies mean?
Test data "reverse"
According to public information, the clinical trial name is Leap-002. medicine.
However, according to the results, compared with the treatment of Levitinib alone, clinical data such as Paborzab's general survival period have improved clinical data such as Paborzab+Lepetinib the treatment of patients in patients, but the results are not statistically significant. Such a result presents "Waterloo" compared with the previously released clinical trial data.
In 2019, at the American Cancer Annual Meeting (AACR), Meridodon announced the results of the phase IB clinical trial. The "Cola combination" was used to treat the objective relief rate of patients with patients with advanced liver cell carcinoma (UHCC) for 42.3%. No progressive survival is 9.7 months. Since then, Meridon has updated clinical data, and the median total survival of patients has reached 22 months, which is much higher than that of Lepartoni. According to the clinical trial named Reflect, the objective relief rate of Levari single drugs is 24%, and the median total survival period is 13.6 months.
Because of the prominent data, the "Cola Combination" was used in the advanced stage of treatment of hepatocytomal carcinoma to be certified by Breakthrough therapy by the US Food and Drug Administration (FDA) in July 2019.
After this published clinical data, Dr. Gregory Lubiniecki, Vice President of Merck Research Laboratory, said in public news that the joint clinical development project aims to solve some of the most challenging cancer treatment types (such as liver cells Cancer) Uncomfortable needs. "Based on a large amount of evidence we have seen so far, we are still confident in the potential of this combination and will continue to study its role in a variety of cancers," Lubiniecki said.
Why is Lepetinib?
The reason why Meridon tests the liver cell carcinoma with the "Cola combination" is from the huge patient group on the one hand. The incidence and mortality are ranked fourth and second in malignant tumors, respectively. Hepatocytate (HCC) accounts for 85%-90%of primary liver cancer, and most of the patients with HCC are in the middle and late stages.
On the other hand, at present, both domestic and foreign pharmaceutical companies are promoting immune combined medication solutions, and local treatment (such as Hengrui Pharmaceuticals (such as hepatic arterial chemotherapy) and TACE (such as hepatic arterial chemotherapy embolism) Compared with the comparison of TACE combined with Corrizumab and Apatinib to simply use the Phase III clinical study of patients with irregular hepatocytoma), it is expected to solve the problem of limited effectiveness of single drugs.
At present, there are two major programs for the first -line treatment of liver cell carcinoma, "T+A" (Roche's Adizumab+Bevarzumab) and "Double Da Da combination" +Bevarzumab) has been approved by the State Drug Administration (NMPA) for the first -line treatment of non -removed or metastatic liver cell carcinoma that has not been removed in the past.
The "Cola Combination" combines targeted drugs and PD-1 immunotherapy. Xu Gang, deputy director of the Department of Cancer Department of Beijing Cancer Hospital, said in response to patient consultation that he often treats Levitinib and PD-1 immunohistos in clinical practice. The efficacy is better than the single use, and the patient's survival is extended. Levitinib can inhibit kinase, thereby promoting anti-tumor activity of Anti-PD-1.
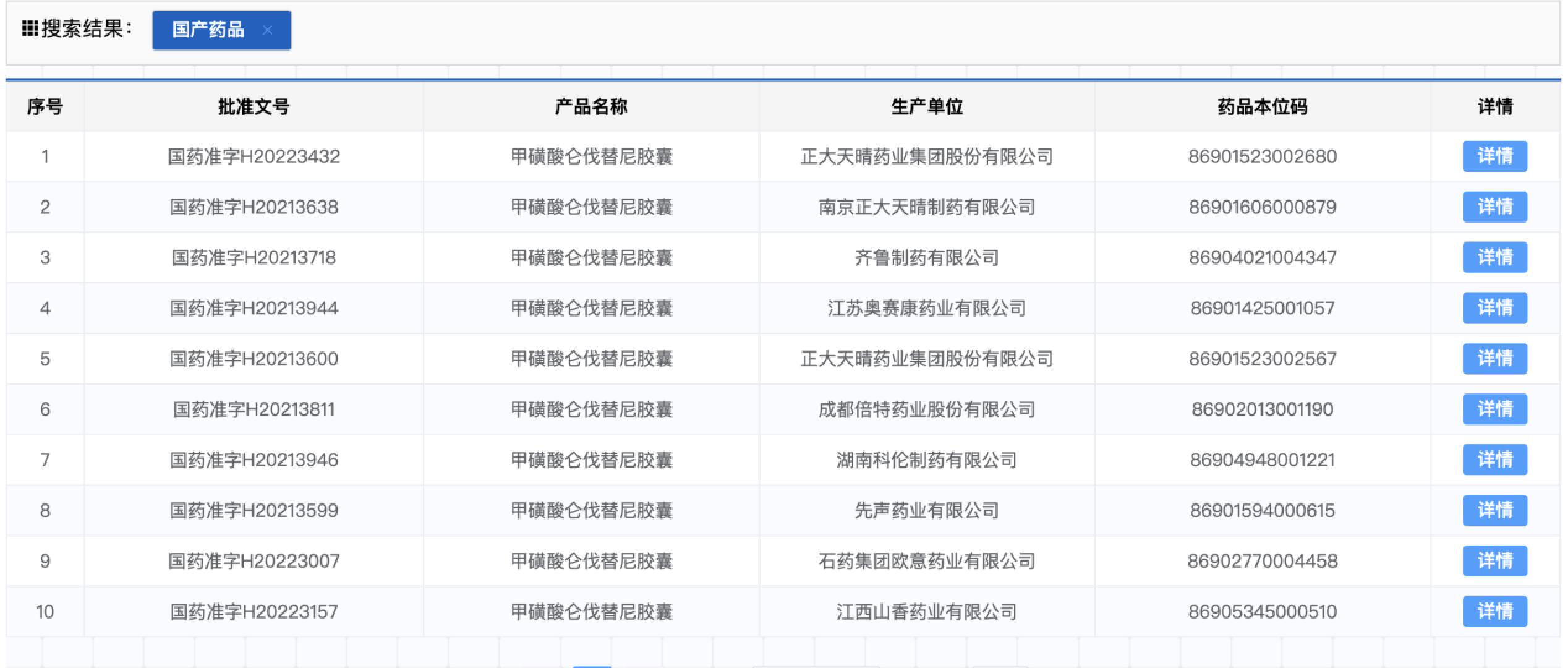
Screenshot of the State Drug Administration
At the same time, compared to the price of Bevarzumab, the price of Lavetinib is lower.
In September 2018, Lavetini was approved in China. At that time, it was priced at 16,800 yuan/box, 30 capsules per box, and 560 yuan per capacity. In 2020, the price of Levitini -based drugs was reduced by 80.7%into the medical insurance directory.
Since then, according to the State Drug Administration, many domestic pharmaceutical companies, including Zhengda Tianqing, Qilu Pharmaceutical, Xianyin Pharmaceutical, Stone Pharmaceutical Group, etc., have been approved to produce Levarini generic drugs. Recently, the seventh batch of centralized procurement of pharmaceutical drugs in Nanjing was opened in Nanjing. Seven generic drug companies won the bid for Levitinib, with an average of 18 yuan per drug. The price of each medicine is 3.2 yuan.
Many pharmaceutical companies start clinical trials
At present, for the adaptation of liver cell carcinoma, many pharmaceutical companies are conducting a joint test of its PD-1 and Lepetinib, or will further open a new market for PD-1.
According to the official website of the State Drug Administration, Roche is carrying out the random and open stage III of patients with "T+A" therapy for patients with "T+A" therapy for the treatment of "T+A" therapy. Research.
The Junshi creatures are conducting clinical trials of Tripley Mippling injection (JS001) or placebo combined with Lepartoni to treat advanced liver cell carcinoma.
Zhongxin Jingwei noticed that Tripley Metropolitania injection was an important item for Junshi creatures, but its sales revenue in 2021 declined. According to the annual report, the annual sales revenue of Tripley Meticuke's injection was 412 million yuan, a year -on -year decrease of 60%. Junshi Biological said that the commercialization of domestic market PD-1 products is becoming increasingly fierce, and Tripley Mippling only has small indications to be included in the national medical insurance catalog, and the larger adaptation of the applicable population has not been approved to be listed. As a result, Junshi Biological is actively promoting the Tripley Meticuketure injection more indications. In mid -June, Junshi Biological announced a planned fund -raising plan of no more than 3.969 billion. Among them, it is planned to use the raised funds of 860 million yuan to invest in Tripley Monkey Monopia's subsequent domestic and overseas clinical research and development.
In the subsequent response to the Shanghai Stock Exchange inquiry letter, the Junshi Bio also stated that in the layout of the R & D pipeline of the anti -tumor product, large -molecular drugs should be centered on Tripley Mipide, supplemented by small molecular drugs, and actively explore joint drugs.
Screenshot comes from Junshi Biological reply letter
Kangfang Bio has a clinical trial of Kadinilian Mipide (AK104, commodity named Kaitini) single medicine or combined with Lezetinib to treat advanced liver cell carcinoma.
According to public information, the Kadinilia injection was approved by the State Drug Administration on June 29 this year, becoming the first domestic double resistance to the listing, and it is also the world's first PD-1/CTLA-4 dual resistance. It is suitable for the past It receives the treatment of recurrence or metastatic cervical cancer patients with platinum chemotherapy.
Kangfang Bio has high hopes for the commercialization of Kodinili's anti -injection. On the one hand, it actively promotes the commercial potential of the indications of Kadonilia to broaden other cancers. On the other hand, Kangfang Bio has established a business team of more than 500 members and will be extended to 800 in the 2022 fiscal year.
Cornerstone Pharmaceuticals is developing the III study of the CS1003 injection combined with Lepetininib to compare the hepatocytal carcinoma.
Generally speaking, on the one hand, Merhadon's folding halberd gave other pharmaceutical companies to warn. Even if it survived the first and second phase of clinical trials, "Waterloo" may appear in the third phase; on the other hand, PD-1 not only price " Inner rolls, the indications are also "inner volume". After Merck's "letting go", the pharmaceutical company is waiting for breakout. (For more report clues, please contact Wang Yuling, the author of this article: [email protected]) (Zhongxin Jingwei APP)
(The views in the article are for reference only, do not constitute investment suggestions, have risks in investment, and need to be cautious to enter the market.)
Copyright Copyright Copyright, without written authorization, no unit or individual may reprint, extract or use it in other ways.
Editor in charge: Li Zhongyuan
Pay attention to the official WeChat public account of JWVIEW (JWVIEW) to get more elite financial information.
- END -
On August 10, the details of Gansu 1+6 (both in Zhangye City) were announced!
August 10, 2022, the epidemic situation of Xin Guan Guan Pneumonia in GansuThe inf...
Preservation of stomach health | Nourish qi is commonly used for you? Which one is suitable for you?

Spring sleepy summer is tired of hibernation, and there are always a group of peop...