Nuggets Innovation Pharmaceuticals | Zezhang Pharmaceutical Double Anti -Drug Clinical Declaration Further Further Fudan Zhangjiang another ADC drug clinical application was accepted
Author:Daily Economic News Time:2022.06.16

"Nuggets Innovation Pharmaceuticals" was jointly launched by the Daily Economic News and Data Data. It aims to interpret the progress and trend of new drug research and development, analyze product competitiveness and market prospects, insight into pharmaceutical capital context, and witness the high -quality development of the pharmaceutical industry.

According to the pharmaceutical data, from May 30 to June 12, 2022, the State Drug Administration of Drug Administration (CDE) received 11 chemical new drugs submitted by 10 listed companies (including listed companies holding companies) Application for new drugs for biological products.

New drug application
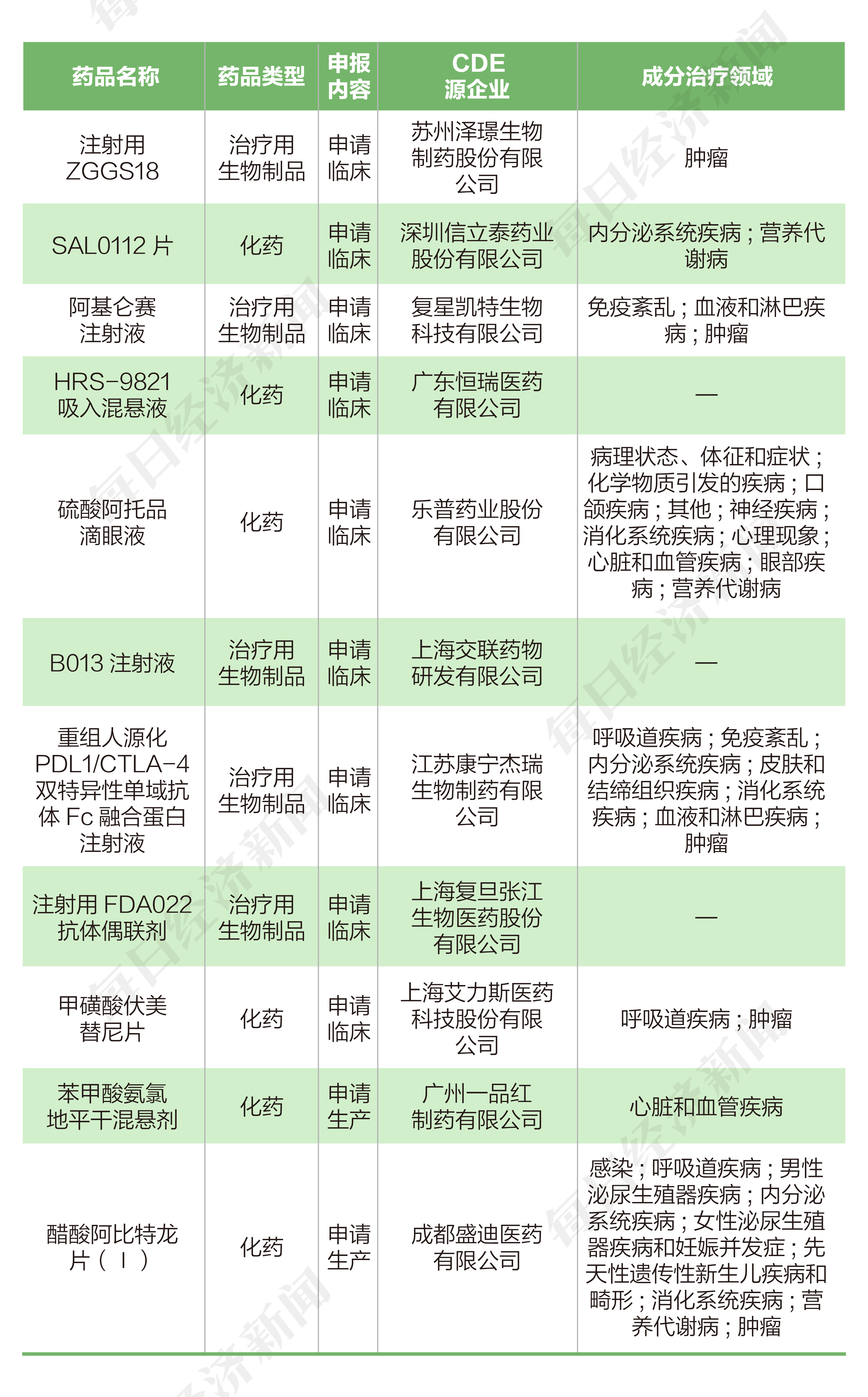
From May 30th to June 12th, 2022, aspects of listed companies, Zezhe Pharmaceutical, Xinlitai, Fosun Pharmaceutical, Hengrui Pharmaceutical, Lepu Medical, Shanghai Medicine, Corning Jerer Pharmaceutical, Fudan Zhangjiang and Aili Li Li Srioli applied for a clinical application; Yipinhong and Hengrui Pharmaceutical each declared 1 production application.
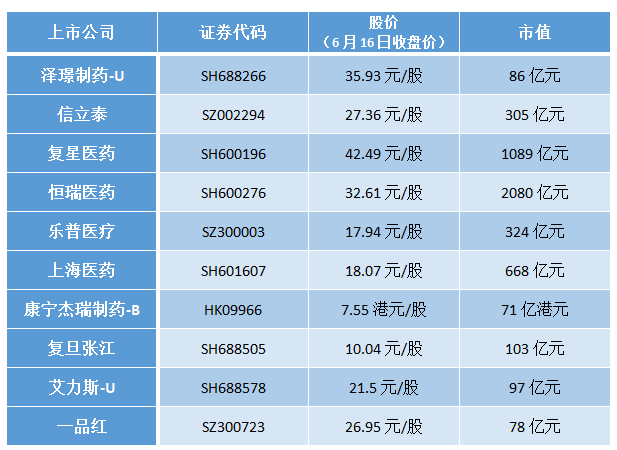
Data source: wind
New medicine hot review
1. Zezhe Pharmaceutical VEGF/TGF-— dual anti-dual-resistant first declared clinical system company 2 to apply for clinical dual anti-drugs
On June 10, Zeye Pharmaceutical announced that the company's independently developed injection was used for the treatment of clinical trials for the treatment of advanced physical tumors for the treatment of advanced physical tumors. According to the company, ZGGS18 is a dual-function antibody fusion protein that is reorganized humanized anti-VEGF/TGF-β. It can specifically combine blood vessel endothelial growth factor (VEGF) and "capture" conversion growth factor-β (TGF-β) (TGF-β) It plays a multi -role role of inhibiting tumor growth in which the newborn vascular formation and reducing the occurrence of tumor metastasis.
Zeye Pharmaceutical also said that ZGGS18 can also improve and regulate the micro-environment of the tumor, which can combine tumor killing effects with tumor immunotherapy drugs such as anti-PD-1/L1 antibodies; pre-clinical research results show that ZGGS18 in human non-small cell lung cancer cancer , Models such as colorectal cancer have significant tumor inhibitory effects, and after combined with Anti-PD-1 antibody, it can cause a significant proportion of mice tumors to completely subscribe, indicating that ZGGS18 has a strong tumor killing effect and enhances tumor immunity The potential for the treatment of drugs.
Industry insight:
Double antidote is considered a new star in the next generation of antibody drugs. It is also one of the most mainstream development directions of immunotherapy drugs. The market potential is huge. Many domestic innovative drug companies have layout. The Head Leopard Research Institute predicts that China's dual anti -anti -industry market will reach 5 billion yuan by 2024.
ZGGS18 targeted VEGF/TGF-β of ZGGS18 of Zezhe Pharmaceuticals. Pharmaceutical data shows that drugs with the same mechanism at present have been approved to go public at home and abroad to enter clinical research. It is reported that ZGGS18 is a dual anti -drug application for clinical trial applications for the company's second paragraph. In January of this year, the company targeted PD-1/TIGIT's dual-anti-drug ZG005 was approved by clinical clinical. The company said that ZG005 is expected to become a tumor immune innovative biological product that treats solid tumors, which is expected to treat a variety of physical tumors.
Company comment:
Established in 2009, Zezhe Pharmaceutical is a newly innovative pharmaceutical company. It landed in the Science and Technology Board in January 2020 with a net raised funding for 1.908 billion yuan. From the perspective of pipeline layout, the company has layout of various treatment areas such as tumor, bleeding and hematological diseases, hepatobiliary diseases and immune inflammation. As of the end of 2021, the company had 16 mainly in the research projects mainly in research projects, and 4 of them were in the NDA, III stage or registered clinical trial stage in the 8 adaptation certificates of drug research.
Since its establishment, Zezhe Pharmaceutical has not yet achieved profitability. From 2019 to 2021, the company has lost more than 1.2 billion yuan in three years. In June 2021, the company's first innovative drug Danutic tablet was approved for listing. The indication certificate was first -line treatment of advanced liver cancer. The first year of listing obtained drug sales revenue was 163 million yuan, which contributed most of the revenue to the company. Donafini has become one of the preferred targeted drugs recommended by clinical guidelines in the field of advanced liver cancer. In the future, the growth of sales volume is worth looking forward to. In addition, the company's R & D expenses in 2021 were 509 million yuan, an increase of 62.13%year -on -year.
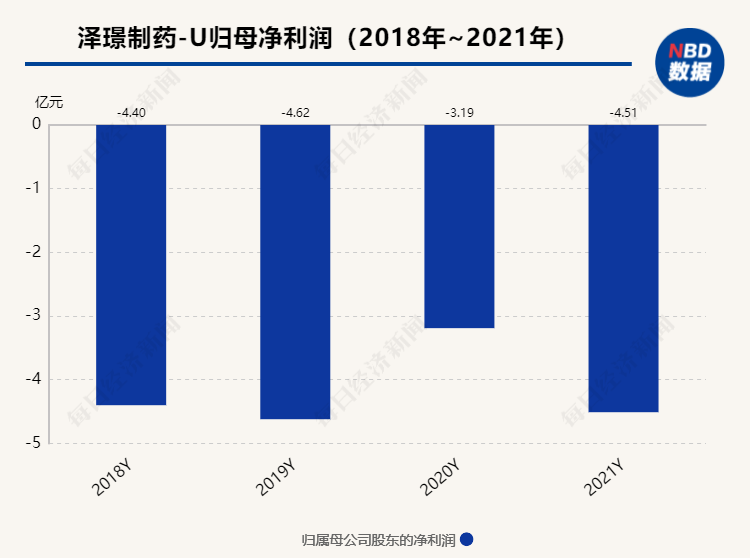
After many years of losses, Zezhe Pharmaceutical is ushered in the harvest period. In May of this year, the company announced that the company submitted the application for the listing of the listing of biological products for the external reorganization of human coordinases. The clinical data of the drug showed that the hemostatic effect was good and had the potential to be widely used in surgery. On June 1st, the company announced that the company's clinical trial application for the treatment of a new type of coronary virus pneumonia patients for the treatment of heavy -duty new coronary virus pneumonia was used for acceptance. The company said that Jackinib may also have the effect of blocking new coronary viruses into patients' alveolar cells, thereby reducing the virus load in the body.
"Nuggets Innovation Pharmaceuticals" researcher believes that under the years of continuous research and development investment, Zezhe Pharmaceutical has made a number of milestones progress. At present, it is entering the harvest period. The company has increased the construction of the commercial team of Donepini, and its capacity growth is worth looking forward to. It is expected to continue to contribute to the company's continuously increasing performance. The local hemostatic drug market for 100 million yuan brings new choices, which is expected to distinguish a considerable market share.
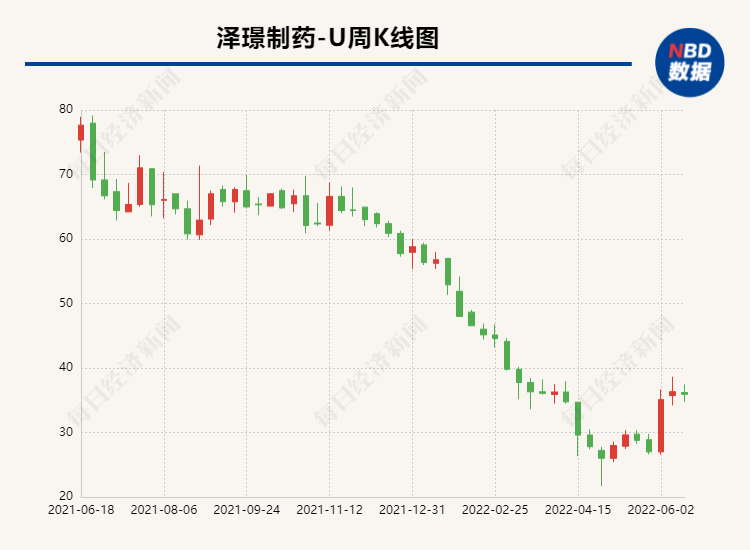
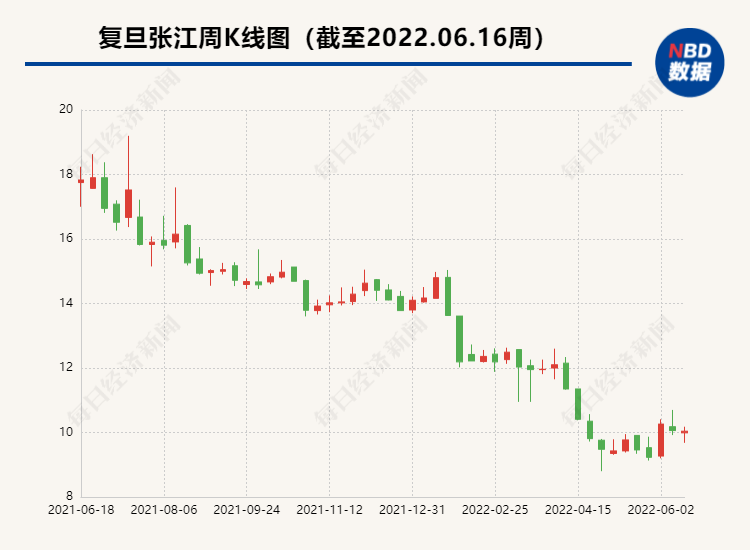
From August 2020 to the present, Zezhe Pharmaceutical's stock price is in a state of falling. At present, the higher stock price has fallen by 70 %. If the new crowded card of Jack Hydrochloride and the clinical trials of other research projects is progressing smoothly, it may promote the company's stock price to recover. 2. Fudan Zhangjiang another ADC drug clinical application was accepted by acceptance of ADC drugs at the ASCO Annual Conference this year
On June 9th, Zhangjiang, Fudan, announced that the company's injection for FDA022 antibody coupling agent (that is, anti -HER2 antibody puppet couplet BB05, hereinafter referred to as FDA022) for the treatment of pharmaceutical I for the treatment of advanced physical tumors was accepted. The company said that in recent years, the company has built the Linker-Drug platform with independent intellectual property rights on the small molecule. FDA022 is the first new generation of ADC drugs on the platform. The antibody and BB05 are composed.
Fudan Zhangjiang also said that FDA022 can swallow and swallow in inside through the combination of tumor cells expressed in HER2, and to cut into small molecular cytotoxic drugs (topological isozyme i inhibitors) to kill tumor cells through protease, kill tumor cells. It is intended to treat HER2's positive advanced physical tumors, such as breast cancer, gastric cancer, lung cancer, and colorectal cancer. The company quoted relevant data that there are currently three HER2 target ADC products that have been listed.
Industry insight:
At the just -concluded Annual Meeting of Clinical Oncology (ASCO), ADC drugs have attracted much attention and become the focus of the annual meeting. ADC drugs refer to a complex composed of monoclonal antibodies that connect cytotoxic drugs to the monoclonal antibody that target tumors. Because of the strong lethality of small molecular drugs and the targetedness of monoclonal antibodies, it has become a jump in the past ten years. R & D hotspots of tumor targeted therapy.
According to Southwest Securities statistics, since the world's first ADC drugs were approved in 2000, a total of 14 ADC drugs in the world have been approved in the world, and ADC drugs have been approved densely since 2019. A total of 9 drugs have been listed, exceeding the previous two previous two two previous two, which exceeded the previous two previous two. The sum of the drugs for ten years. Benefiting from the gradual expansion of ADC drugs and the excellent efficacy, ADC drug sales have repeatedly achieved great achievements. Global ADC sales in 2021 were nearly $ 5 billion, and from 2014 to 2021, the compound growth rate was about 30%.
At present, the competition for the R & D of ADC drugs has become increasingly fierce. Domestic and foreign pharmaceutical companies have unanimously optimistic about the development potential of ADC drugs, and have increased the tracks. According to Southwest Securities statistics, Chinese companies have adopted ADC drugs such as independent research and development, technology introduction and product introduction. There are more than 170 ADCs in China, of which nearly 60 have entered the clinical stage. At this year's ASCO Annual Conference, the domestic ADC pioneer Rongchang Bio (SH688331, the stock price of 39.7 yuan, a market value of 21.6 billion yuan), Lepu Bio-B (HK02157, HK $ 6.9, market value of HK $ 11.5 billion), Caren Pharmaceutical (SZ00242222 The stock price was 18.83 yuan, and the market value of 26.8 billion yuan) has announced the latest research results of its ADC products. Among them, the Vidici monocity of Rongchang Bio is the first approved domestic ADC product, which has been approved by the two major indications of gastric cancer and urinary epithelial cancer in China last year.
From the perspective of target layout, HER2 is currently the number one target for global ADC drugs. According to Medicine Rubik's Cube data, as of June 2022, there are 60 ADC drugs that have been listed or developed in the world. The FDA022 target for the clinical FDA022 of Fudan Zhangjiang is also HER2.
Company comment:
Fudan Zhangjiang is an old -fashioned pharmaceutical company. It has been established for more than 20 years and is also a company listed on the "A+H". The company mainly covers the two major areas of skin diseases and anti -tumor. At present, the treatment of Ella, HPV infectious diseases and proliferative diseases represented by condyloma acuminatum, Ella in the treatment of tumors, and compound beauty for the treatment of bright red spots. The company's three most important products contributed 99.6%of the revenue of the company's sales of medicine and diagnostic products in 2021. The above three drugs were approved for listing and sale earlier, in 2007, 2009 and 2017, respectively.
In 2021, Fudan's revenue and net profit returned from Zhangjiang were 1140 million yuan and 213 million yuan, respectively, which basically returned to the level of 2019 before the epidemic. However, since landing in the Science and Technology Board in June 2020, the company's stock price has been in a state of overcast falling, and the current higher stock price has fallen by most of them.
The ADC drug structure is complicated and the development is difficult. The technical platform is the primary barrier of ADC pharmaceutical companies. For Fudan Zhangjiang, ADC drugs are an important R & D direction of the company's genetic engineering technology platform at this stage. It already has two ADC drugs in the clinical trial stage. "Nuggets Innovation Pharmaceutical" researcher believes that HER2 target research and development competition is extremely fierce, and currently 3 HER2 target ADC drugs have been approved to be listed. For FDA022, which has just been declared clinical, it is destined to accept R & D and commercialization. Double challenge. However, the company's current three core products are still in the growth of volume, and it is expected to provide stable cash flow for the company's R & D investment.
Daily Economic News
- END -
State Drug Administration: 10 batches of drugs of 9 companies do not meet the regulations
According to the official website of the State Drug Administration, after the insp...
As the saying goes | Choose the treatment plan to think about it?Spleen and stomach function is the key!

Open -columnAs the saying goes is the first Chinese medicine department with infla...