What does PFS and OS dual -ended positive mean?Professor Liang Fei takes you from the perspective
Author:Cancer Channel of the Medical Time:2022.08.05
*For medical professionals for reading reference
Professor Liang Fei revealed the mystery behind the Impower133 research design.
In recent years, with the development of immunotherapy, the predicament of the treatment of small cell lung cancer (ES-SCLC) in a wide range has gradually changed. IMPOWER133 is a milestone study in the ES-SCLC field. In the study, the first-line therapy for the first-line ES-SCLC of the Ayidozumab and the EC (EC) to obtain the total survival (OS) and the non-progressive survival period (PFS) Double benefits. Based on this, Ayidarzumab combined with the United States, China and many other countries have been approved by the ES-SCLC first-line treatment certificates, so that SCLC officially enters the era of immunotherapy. At the same time, with the announcement of research data such as Caspian, ES-SCLC immunotherapy ushered in more breakthroughs.
In this regard, Professor Liang Fei of Zhongshan Hospital affiliated to Fudan University invited Professor Liang Fei, affiliated to Fudan University to interpret the characteristics of Impower133 in the perspective of statistical science. SCLC front -line treatment optimization option to provide reference.
IMPOWER133 Review: The first ES-SCLC front-line immunotherapy obtains PFS and OS dual-end-dual point positive
IMPOWER133 is a global, double -blind, placebo -controlled phase III study. Study in 403 patients with ES-SCLC who have not been treated, according to 1: 1 randomly allocated to receive 4 cycles of Adizumab+chemotherapy or placebo+chemotherapy induction treatment, and then continue to use Adi Zhuzumab or comfort comfort Maintenance treatment until an irisiated toxicity, progress or no clinical benefits.
It is worth mentioning that, unlike other SCLC immunotherapy studies, the study uses PFS and OS dual -end -point design.

Figure 1. IMPOWER133 Research Design
Professor Liang Fei pointed out that although OS is the gold standard of clinical studies, the end point of clinicians and patients, and the end point for the drug supervision institution. But in fact, PFS also has very important clinical significance. It can evaluate whether the treatment plan can delay tumor progress and improve the quality of life of patients.
IMPOWER133 studies the design of OS and PFS dual -end points, which has higher statistical requirements. Two key requirements are: reasonably allocate the alpha value between different research end points; calculate the sample amount for each research end point to ensure sufficient statistical academic effectiveness to confirm the conclusion.
For phase III clinical studies, especially the critical research of confirmed registration, the overall α value is only 0.05. If you use double -end points, it means that the α value of the initial allocation of each end point will be smaller, so the difficulty of success each endpoint is larger than the end point. Because of this, if the dual -end point can be achieved at the same time, its clinical significance may be more important.
Moreover, from the perspective of statistical perspective, only by participating in the research end of the alpha -value allocation and calculating the sample volume can we give confirmation conclusions and support the approved for the application. The benefit claiming the pharmaceutical manual.
The double -end point design of the IMPOWER133 studies meets the above requirements. Study the overall 0.05 α between OS and PFS, and the initial scores of OS and PFS are divided into 0.045 and 0.005, and the α recycling strategy is used. At the same time, from the perspective of samples, for the end of OS and PFS, the IMPOWER-133 studies have sufficient conclusions under the confirmation of statistical academic effectiveness. The positive conclusion of the PFS results obtained in this case is undoubtedly more credible.
With the excellent efficacy of Ayidarzumab, IMPOWER133 finally lived up to expectations. Both endpoints obtained the requirements of statistical sciences, reaching the preset end point, and both the highest level of evidence.
In 2018, "New England Medical Magazine" published the IMPOWER133 research results showing that when the median follow -up was 13.9 months, the median OS of the Adelin Mipido group and the comfort unit was 12.3 months and 10.3 months (HR = 0.70 , 95%CI: 0.54-0.91; P = 0.007), the median PFS is 5.2 months and 4.3 months (HR = 0.77; 95%CI: 0.62-0.96; P = 0.02) [1]. Due to the very good efficacy, OS and PFS have reached positive results during the mid -term analysis, and the IMPOWER133 research ended in advance.
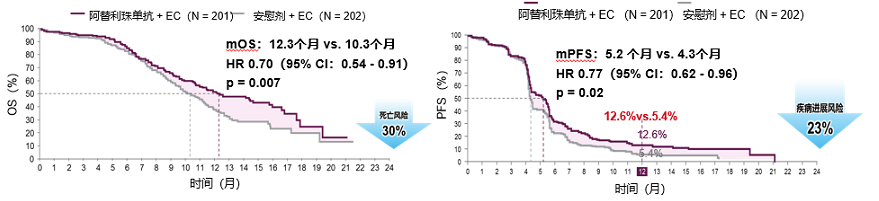
Figure 2. IMPOWER133 Study mainly OS and PFS results
The IMPOWER133 research is the first successful global multi -centered study in the SCLC field. It is also the only clinical study that is currently the only OS and PFS dual -end dual points, which has set a benchmark for subsequent clinical studies.
How to choose different treatments? The stability and credibility of the result are important considerations
ES-SCLC's first-line treatment has been announced by many studies. However, due to the lack of head opposite comparison, it is impossible to make a clear judgment on the efficacy of different drugs.
Professor Liang Fei pointed out that in this case, clinicians need to use some indicators to exploratory judgments, including median, HR values and OS rates when they are treated with some indicators. Among them, the HR value reflects the difference between the two survival curves, so it is more comprehensive and stable. The HR value is the focus of clinical research, and it is also an important indicator for the benefits of drug benefits in the European Cancer Internal Science Society (ESMO) and the American Clinical Oncology Society (ASCO) clinical beneficiaries. The degree of improvement of median values and OS rates are the easier to understand indicators of clinicians, but it is worth noting that the median and OS rates in the research report are usually estimated and have a certain instability. If the follow -up time is not enough, or the data maturity is low, at this time, it can also estimate the median value and the OS rate of 1 year or 2 years. However, if there is the following situations, if the median follow -up time is shorter than the median value, the 95%confidence range is wide, or the number of risk people (NO. AT RISK) as of the mid -range follow -up time is far less than half of the total number of tested groups. It means that the median value may not be stable enough. With the extension of the follow -up time, the median value and OS rate may change, and even in some cases changes significantly.
Therefore, when the clinician interprets the median value and OS rate, it should also pay attention to the comprehensive judgment of the confidence interval, the maturity of the data, and the medium follow -up time according to the stability and credibility of the result. For example, in the IMPOWER133 study, the median OS of the Adelicopic Mummy group was 12.3 months, and the median follow -up time has reached 13.6 months, so the median OS results are very stable.
SCLC immunotherapy research future exploration direction prospects
Although immunotherapy has changed the entire SCLC first -line treatment model, compared with non -small cell lung cancer, the overall prognosis of SCLC patients is still relatively poor.
Professor Liang Fei said that the biggest problem of current SCLC immunotherapy is how to further improve the efficacy on the basis of immunochemical chemotherapy. In the future, more combined treatment modes such as immune+chemotherapy+other drugs, immune+chemotherapy+radiotherapy should be explored clinically. crowd.
In order to solve these problems, in addition to carried out clinical trials, clinicians can also conduct preliminary exploration and analysis of different therapeutic plans and biomarkers in clinical practice through real -world research. If it is prompted to benefit, you can further carry out one -arm research and small sample random control clinical research. Based on this, a clear conclusion is obtained through multi -center large -scale III clinical research.
In the future, I look forward to more evidence -based medical evidence such as Impower133 to study, so that more SCLC patients will benefit from immunotherapy.
Expert Introduction
Professor Liang Fei

Zhongshan Hospital of Fudan, Biological Statistics Office, Statisticser
Member of Fudan University Evidence -based Medical Center
Member of the Shanghai Anti -Cancer Association Cancer Prevention and Screening Committee
CSCO Youth Committee Statistics Group member
With the first author (including a total) or communication authors in JCO, Annals of onCology, JNCI, EUROPEAN JOURNAL of Cancer and other magazines published 15 SCI on SCI, the cumulative influence factors exceeded 150
With the first author in Nejm, Lancet, Lancet Oncology, JCO Published Letter 8
JNCI, Clinical Cancer Research, Theranostics reviewer
As a clinical study initiated by a statistician participating in dozens of researchers, the relevant results were published in JCO, Annals of Surgery and other magazines
Main research directions: clinical trial design and statistics, clinical research methodology
references:
[1] Horn l, masfield as, szczęsna a, et al. FIRST-LINE ATEZOLIZUMAB PLUS Chemotherapy in Extensive-Stage Small-Cell LUNG CANCER. N English J Med. 2018 DEC 6; 379 (23): 222022222222222222222222222222222222222222222222222222
*This article is only used to provide scientific information to medical people, and does not represent the viewpoint of this platform


- END -
The maximum drop of medicines is more than 90%!"Double Channel" in Linyi County, Linyi sent "life -saving medicine"
I did not expect that in the county, you can buy sulfonic acid mulpinibinib, and you no longer have to go to the city to get medicine. This is really a time to us with a slow disease patient! In a l
High temperatures and alert radical diseases in the next day: Different from heat stroke, the mortality rate is higher
Different from heat stroke, the mortality rate is higher--High temperature for several days, alert thermal radiation diseaseAt 14:30 on July 13th, the temperature of the Shanghai Xujiahui Station reac