About 1 billion pieces of annual output!Real biology intends to go public in Hong Kong
Author:Dahe Cai Cube Time:2022.08.05
[Dahe Daily · Dahecai Cube] (Reporter Wang Leibin) Following the production of the new crown oral medicine on August 2, Henan Real Biotechnology Co., Ltd. (hereinafter referred to as real creatures) came again.
The Hong Kong Stock Exchange disclosed on August 4 that the real creature submitted a listing application to the main board of the Hong Kong Stock Exchange. CICC is an exclusive sponsorship.
According to the real biological listing application, the use of the IPO raised funds, including the company's manufacturing and commercialization of Azfdin, the core product of the company's treatment of new crown pneumonia. Wait.

Real creature starts Hong Kong listing
Funding funds for manufacturing and commercialization for Azfdin
Real creature was established in Pingdingshan City, Henan Province in 2012. It is an innovative drug R & D enterprise integrating independent research and development, production and sales. It is mainly engaged in the research and development of innovative drugs such as antiviral, antitumor, cardiovascular, and liver disease.
The application shows that the real biological fundraising funds are mainly used for the manufacturing and commercialization of COVID-19 in the core product Azf; clinical development of candidate drugs before clinical or in the IND stage; Potential acquisitions or introduction of candidate drugs provide funds; used for operating funds and other general enterprises.
It is reported that the core product of the real creature is a pyrine nucleoside drug, which has a broad -spectrum antiviral activity. Earlier, in July 2021 and July 2022, they were approved by the State Drug Administration for the treatment of HIV infections and COVID-19, and the first national drug regulatory bureau developed by the Chinese company was approved to treat COVID-COVID- 19 orally directly antiviral drugs.
At present, real creatures have been fully prepared to start Azfdin's commercial sales.


Autonomous production line can produce 1 billion tablets Azfding
Signed a strategic agreement with a number of pharmaceutical manufacturers
On July 25, Fosun Pharmaceutical Holding subsidiary Fosun Pharmaceutical Industry and real creatures signed the "Strategic Cooperation Agreement" to reach a strategic cooperation on promoting the joint development of both parties and the exclusive commercialization of Fosun Pharmaceutical Industry Azfding.
According to the cooperation agreement, Fosun Pharmaceutical Industry enjoys the exclusive commercialization of cooperative products, and commercialization includes acting, importing, exporting, sales, and promotion.
It is understood that the cooperation areas of real creatures and Fosun Pharmaceuticals include new crown virus, AIDS therapy and prevention.
In fact, in the process of real creature Azf's research and development, many listed companies have issued announcements to cooperate with them.
In April of this year, the real creature and Xinhua Pharmaceutical signed agreement, agreeing that Xinhua Pharmaceuticals as products and operators in China and other countries agreed by both countries as Azfding; in May this year, real creatures signed with China Resources Shuanghe. Related agreements entrust China Resources Shuanghe to process and produce Azf's fixed film.
In terms of raw medicines, the current partner of real creatures is Takinxin Pharmaceutical in Xinxiang, Henan.
According to the real biological listing application, at present, real creatures have entered into a strategic agreement with many leading drug manufacturers (including Beijing Union) to produce and supply Azfdin's active drug components (API) and tablets.
At the same time, real creatures said that the company also has its own production capacity, with an annual production capacity of about 1 billion tablets Azfdin. Based on the combination of ownership and contract capacity, Azf, which can produce enough amounts of Azf to meet market demand.

Two years of loss of 566 million yuan
It is mainly used for R & D investment, capital expenditure and sales expenses, etc.
Financial data shows that in 2020, 2021 and 2022, other revenue and income of real creatures were 68,000 yuan, 1.376 million yuan, and 8.451 million yuan, respectively, while losses of 151 million yuan, 197 million yuan, and 218 million yuan, respectively Essence In two years and 5 months, the cumulative loss of real creatures was 566 million yuan.
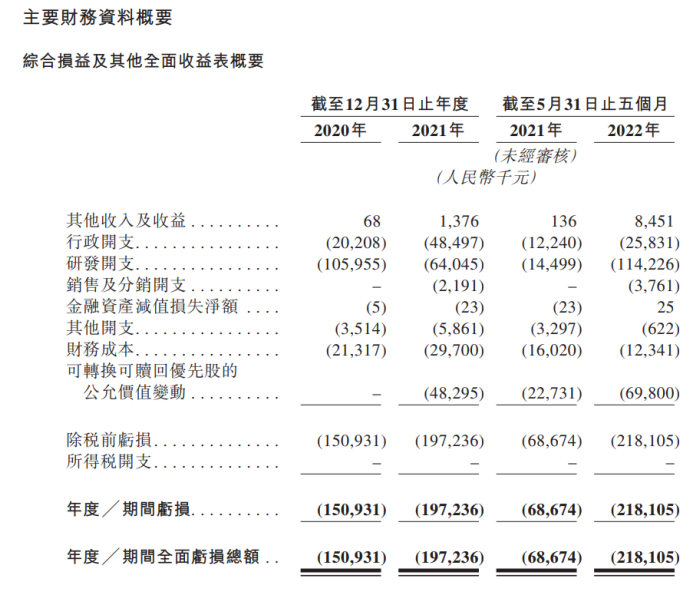
For the cause of losses, real creatures said that they are mainly used for R & D investment, capital expenditure and sales expenses. Among them, in the past two years and the first May of this year, the cumulative R & D expenditure of real creatures has reached 284 million yuan.
As of now, the total real biological stocks are 268 million shares, and the largest shareholders are Sanlian Innovation, holding 46.89%of the shares. Chaoyang, the founder of the company, is one of the controlling shareholders, and controls 48.61%in a direct or indirect way.
At present, real creatures have completed two rounds of financing A and B.
In November 2020, the real creature completed the A round of financing, which was exclusively led by Yifeng Capital, and the financing was used for innovative pharmaceutical technology research and development and market expansion.
In August 2021, real creatures completed a round B financing of 100 million US dollars. This round of financing was led by Yifeng Capital and Yingke Capital. The project registration and the commercialization of Azfdin, which have been approved, and so on.
In addition to the above financing, the establishment of the relationship between Fosun Pharmaceutical and real biological cooperation has also paid a lot of funds for it. According to the cooperation agreement, this cooperation Fosun Pharmaceutical must pay 800 million yuan to the real creature.

Except for Azf
And many innovative medicines are under development
Real creature said that in addition to Azfdin, it is currently actively developing other innovative candidate drugs for treating viral, tumors and cerebrovascular diseases, including CL-197, a oral long-acting purine nucleus for the treatment of HIVAntidin antiviral drug; Demotinib, a third represents the epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI) anti-tumor candidate drugs, compared with the widely used third-generation EGFR-TKIThere is potential to become a more secure and lower effect of toxic and side effects; and MTB1806, a small molecular candidate drug for acute ischemic stroke. By observing in preclinical research in clinical studies, its lower dose -level administration solution can be available.It is comparable to the NBP (AIS drug approved by the State Drug Administration).In addition, there are several other small molecular candidate drugs in clinical clinical stages.
Responsible editor: Chen Yuyao | Review: Li Zhen | Director: Wan Junwei
- END -
Chongqing Zhongxin Cancer Hospital, open consultation!

Yongchuan released a message5 daysChongqing Zhongxin Cancer Hospital opening cerem...
The retired teacher's atresia for 4 years has finally opened up. He said, "Doctor Zhou Yunzhi gave me a second life"
This patient is a retired teacher from the Kazakhs. Four years ago, due to the aft...