Chemical learning -the generality of acid
Author:Teacher Zong Chemical root car Time:2022.08.05
First, the generality of acidity
(1) The role of the pH indicator agent
(2) Reaction with the lively metal reaction (replacement response)
(3) Reaction to certain metal oxides (reaction reaction)
(4) Reaction to alkali (neutralization)
(5) Reaction to certain salt (reaction reaction)
Second, concentrated hydrochloric acid and concentrated sulfuric acid
(1) Concentrate hydrochloric acid
Colorless and irritating liquid, volatile, react with silver solution to test the hydrochloric acid
(2) Concentrated sulfuric acid
The colorless and viscous oil -like liquid, water absorption, dehydration, oxidation, releases a lot of heat when dissolved in water, and reacts with chloride solution to test sulfuric acid root ions.
Third, perceive the generality of sour
The acidic substances we are in contact with are acidic, because the cationic ions from the acid electricity are all hydrogen ions, and the generality of acid is reflected by hydrogen ions. There are generally five aspects of acidity: first of all, acidic can be used with acid -base indicator, such as turning the purple stone reedous test solution, making the colorless phenolon test solution unchanged, etc. Salt and hydrogen; Third, react with alkaline oxides to generate salt and water; fourth is to neutralize salt and water with alkali reactions; fifth is to generate new acids and new salt with salt reactions. When we know, we can put acid on our right hand heart. The five fingers represent the five properties of the five types of substances that react with the acidic response, so that the pioneering of acid is firmly in our right hand. It is very convenient to recognize the generality of acidity!

- END -
Admission process form "please collect! Shandong 2022 college entrance examination enrollment admission work opinion released
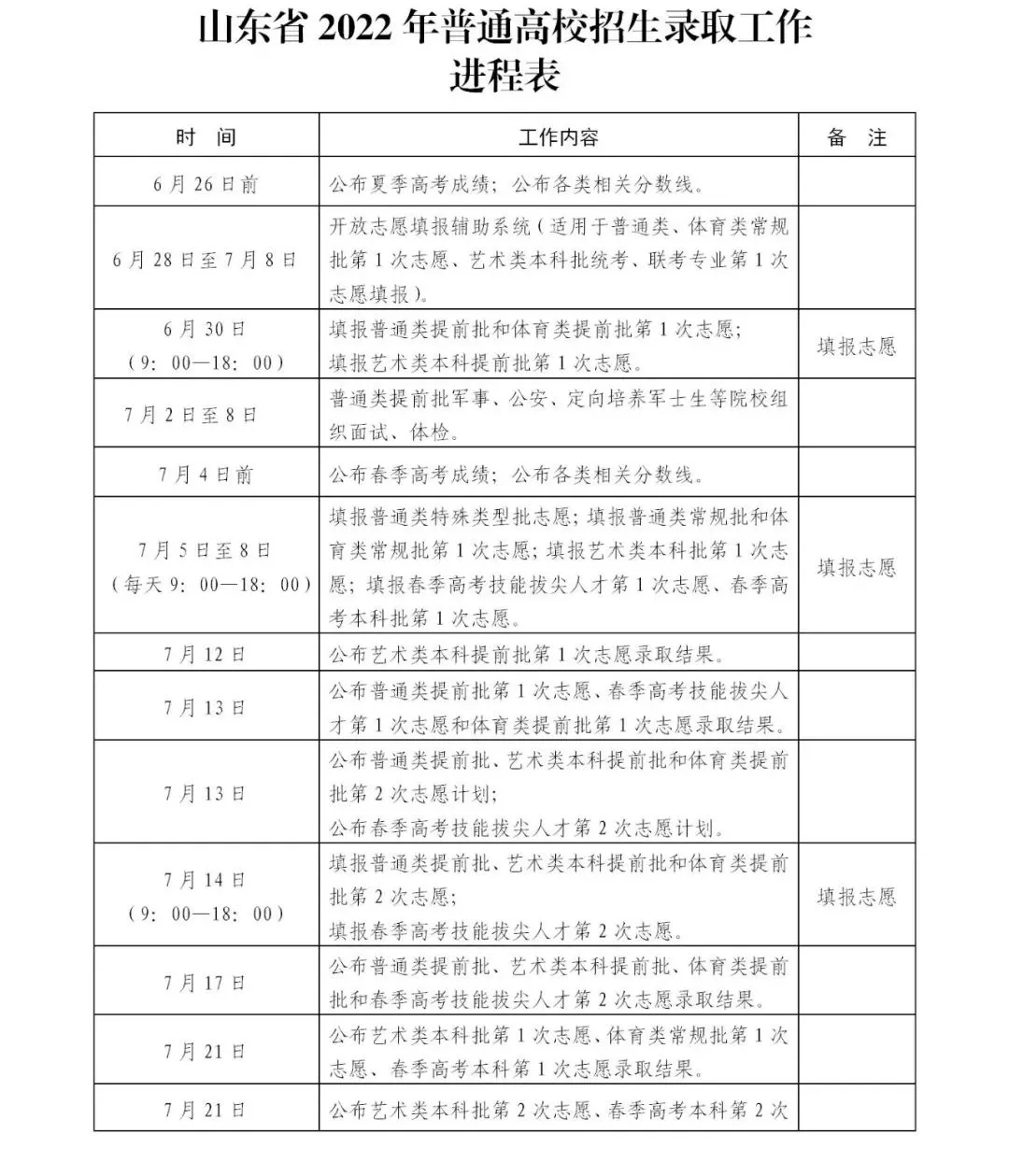
On June 17, the Shandong Provincial Education Admissions Examination Institute iss...
Follow | Yinchuan 4 high school, the enrollment of sports special students is released!

Recently, the WeChat public account of the WeChat public account was released as Y...