Can I eat starch synthesized with carbon dioxide?Frankly, I don’t dare to eat, but I don’t want to eat it.
Author:Look at the think tank Time:2022.09.24


Hello everyone, I am Cai Tao from the Tianjin Institute of Industrial Technology of the Chinese Academy of Sciences. Today I will share with you the story of starch synthesis.
When it comes to starch, the first thing we think of is food. Our global food production consumes nearly 40%of land and 70%of freshwater resources, and also consumes a lot of fertilizer and pesticides. Even so, according to the statistics of the world's grain and agriculture, nearly 1 billion people around the world face serious hunger threats. We do n’t want to underestimate these 40%of the land. This is except the frozen soil of the North and South Pole, the deserts that cannot be a cultivated land, the desert, and the tropical rain forests we can no longer cut. This also means that in the future, we cannot increase the output of grain by large -scale increased cultivated land.
As the global population grows, our demand for food is still increasing. How to deal with this problem? The core challenge here is how we use limited resources, limited land, and limited freshwater to produce more food.
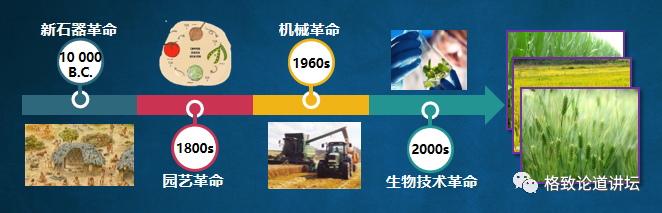
Let's take a look at the paradigm of food production, that is, to produce grain through cultivated land. More than 10,000 years ago, our ancestors actually mastered this method, sowing seeds on the land, and harvesting food in autumn. In modern times, we have experienced a series of technological revolution. These technologies have continuously increased the output of crops, but the paradigm of this spring planting and autumn harvest has not changed. Now we see more and more clearly that this paradigm ceiling is already there.

With a traffic behavior example, what is our most primitive way of traveling? Just walk on our legs. The ceiling of this way is Bolt. If I race with him, I may not even see his shadow. But if I give me a fast horse or a sports car, I may easily surpass him. This is the charm brought by the paradigm change, which can turn the impossible things under the previous paradigm into possible. Now human beings can go to the moon through the transformation of travel methods, and we may go to Mars in the future.

Ma Yanhe, chief scientist of artificial synthetic starch project
Ma Yan, the chief scientist of the artificial synthetic starch project, has made a very vivid metaphor for the incident: the technological revolution of the past agricultural experience "has not been separated from the carbon solidification model of the plant itself. Also rely on your feet. Can you jump out of this model and build a car? "
Literature 丨 Researcher, Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, Cai Tao Chinese Academy of Sciences
This article is reproduced from the WeChat public account "Gezhi Tao Tao forum" (ID: Selftalks). The original first published on September 23, 2022. Can the original title "Can starch synthesize with carbon dioxide be eaten?" Frankly speaking, I don't dare to eat, but I can't bear to eat | Cai Tao ", which does not mean looking at the view of the think tank.
1
Made in thin air? is it possible?
So how do you build "this car"? We must first analyze what the main components of food are.
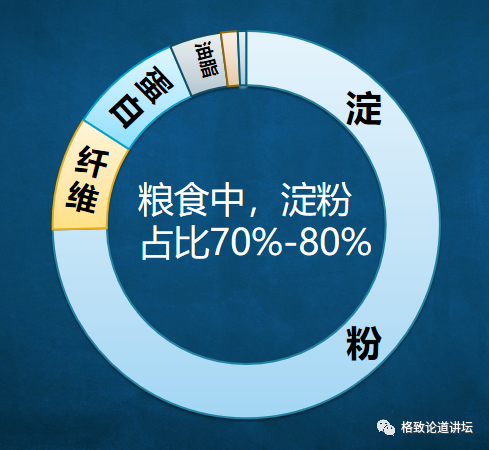
Taking grain food as an example, its 70%-80%ingredients are starch. The global annual food output is about 3 billion tons, of which nearly 2 billion tons are starch. So, is it likely to change a way to produce starch? So in 2015, our research institute officially set up artificial starch synthesis projects. The original intention of this project is to turn the agriculturalization process of starch into a process of industrialization. We call this paradigm "agricultural industrialization".

What are the benefits of doing this? First, we can shorten the long cycle of planting crops. For example, it takes 100-150 days to plant corn, and we can shorten it to 2-3 days. In addition, it can greatly reduce the dependence of agricultural planting to land, and starch can be generated in a small space in the future. More importantly, because this method no longer depends on plants to synthesize starch, it can truly get rid of the dependence on land and water resources and reduce the impact of agricultural planting on them.
At the beginning of this project, we hope to use carbon dioxide and solar energy in the air to directly manufacture starch, so it is named "manufactured out of thin air" for this project. But is it really possible to make it out of thin air?

On the left is a photo of the early project, the person with the second half -face face from the right is me. At that time, I was still a very happy young man, because I haven't realized how severe challenges we will face in the future.
Let's take a look at how to turn starch from agricultural production to industrial production and what challenges we face in this process.
Three challenges of starch industrialized production
Let's first analyze how crops synthesize starch. First of all, crops need to absorb solar energy and turn solar energy into the form of ATP or NADPH that can be used by creatures. However, because the energy density of solar energy is very low, the average of about 1,000 watts per square meter is only 1,000 watts, which means that plants must be planted on a large area to get enough energy.
With energy, you need to fix carbon dioxide. Plants use four ten thousandths of carbon dioxide in the atmosphere, which is a very thin concentration. That means that it takes a long time to accumulate enough carbon source to synthesize plants and our target product starch.
However, fixed carbon dioxide does not mean that starch is generated, and plants need to go through a long and complicated synthetic channel, and this path is very strict regulatory. For example, the corn needs to be used as a whole after the plant grows up, and the corn stick will be knotted under nutrients, and starch will accumulate in the grains of corn. The idea of dealing with the three challenges
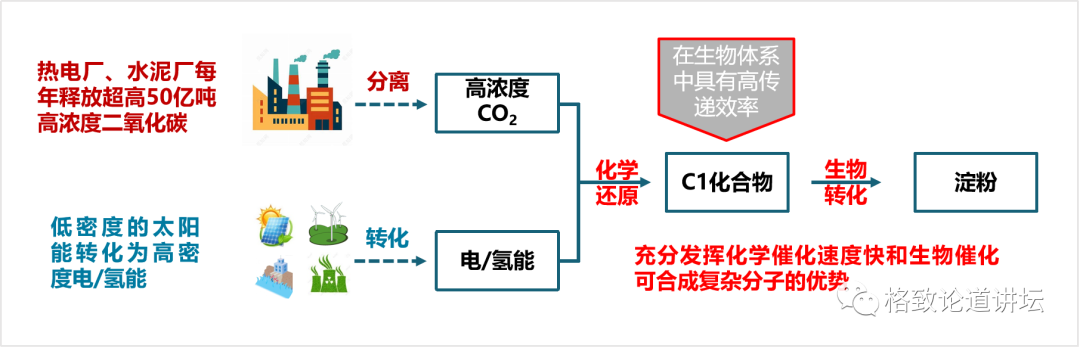
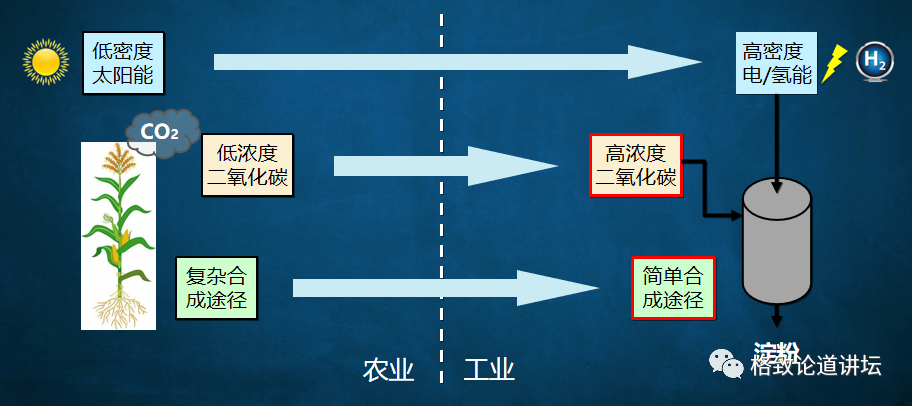
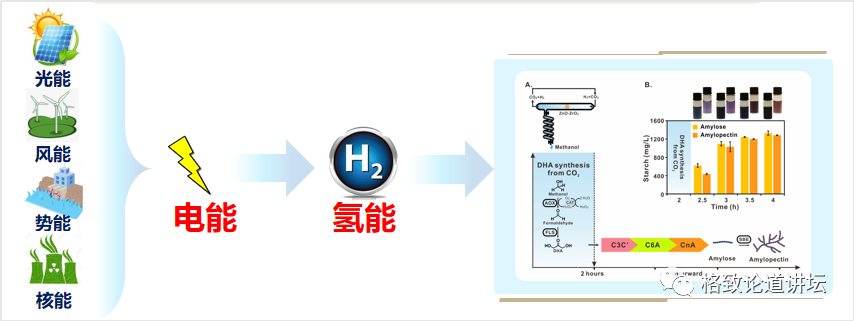
When we want to understand these challenges, we put forward ideas to deal with these challenges. First of all, where does the carbon dioxide come from? The heat power plants and cement plants in our country are fixed sources, with carbon dioxide of about 4 billion-5 billion tons each year. The concentration of these carbon dioxide is greater than 90%, or even close to 100%, and we can catch these high concentrations of carbon dioxide.
On the other hand, we can convert low -density solar energy into high -density electrical energy and hydrogen energy. The energy density can be ten times, 100 times, or even thousands of times of solar energy density. In addition to using solar energy, wind, water, and even nuclear energy can be used. Our country's recent nuclear aggregation has made breakthroughs in succession. If this technology is really applied, then there will be inexhaustible and inexhaustible green energy sources.
Finally, we need to design a process of chemical reduction to transform high -concentration of carbon dioxide into a simple carbon -bearing compound. The carbon -a compound is then transformed through the transformation of the biological process to synthesize it into a very complicated starch molecule. This design idea not only exerts the advantages of fast chemical catalytic speed, but also gives full play to the advantages of biocratricity that can synthesize complex molecules.
2
The first time I saw "starch blue"
Let's take a look at this process of chemistry. In fact, this field has developed for two or three decades, and many technologies have been very mature and even close to industrialization. Carbon dioxide is restored by different energy forms and can generate methic acid, carbon monoxide, methanol and methane.
Carbon dioxide chemistry restoration
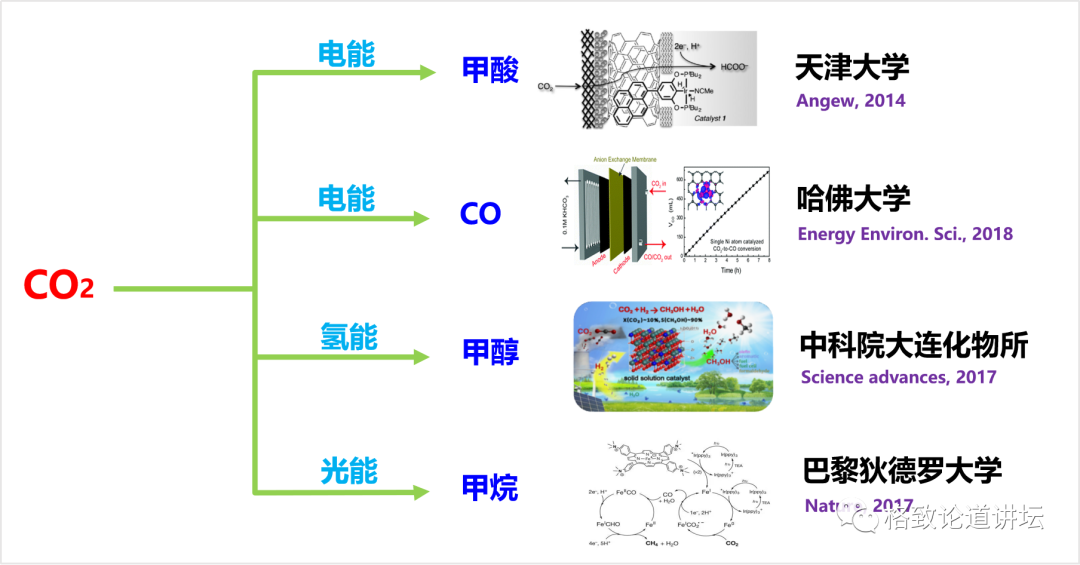
We do not use carbon monoxide and methane because they are gas, and their quality transmission efficiency in the later biological conversion system is very low. The water -soluble of methic acid and methanol is very good, which can greatly improve the efficiency of starch synthesis. Therefore, we chose methalic acid and methanol as intermediate molecules for bridges and chemical reactions.
How to design a simpler starch synthesis approach
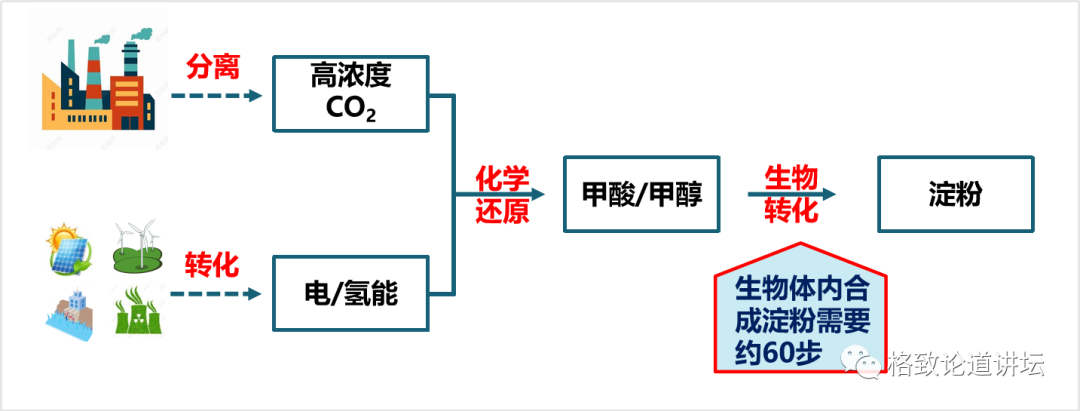
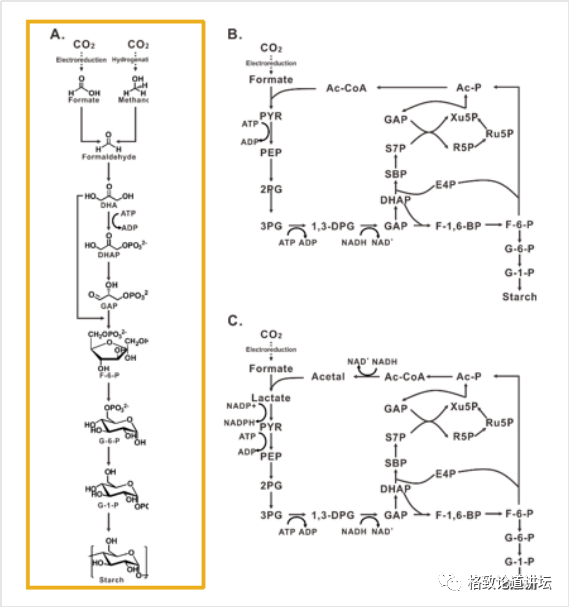
Finally, we have a key job to use the way to calculate the way to make these methalinic acids and methanol into starch. Here we put forward two principles. One is the minimum principle of energy dissipation. Carbon dioxide is a very inert compound. If we want to convert it, we must enter energy. The less the input energy, the higher the energy efficiency of the entire system, and the lower the cost may be. Second, we hope that the shorter the response steps, the better. Because the shorter the response steps, the easier it is to design and operate, the higher the synthetic efficiency.
Through these two principles, we calculate 4 possible starch synthesis paths from more than 6,000 reactions. There are two in Group A, except that most of them are overlapped. Based on calculation, we found that theoretically, theoretically, carbon dioxide to starch only needed 9 steps to react to synthesize. We were very excited at the time. But can it truly achieve it?
Here we want to introduce a catalyst -enzyme in biological science. Taking this reaction as an example, there are two tri -carbon compounds, one is called phosphate dihydroxylcetone, and the other is called triggeosylcetaldehyde. Under natural conditions, these two compounds will aggregate a six-carbon compound called 1,6-two-phosphate. But this process has taken place very slowly, and it may take millions of years to complete.
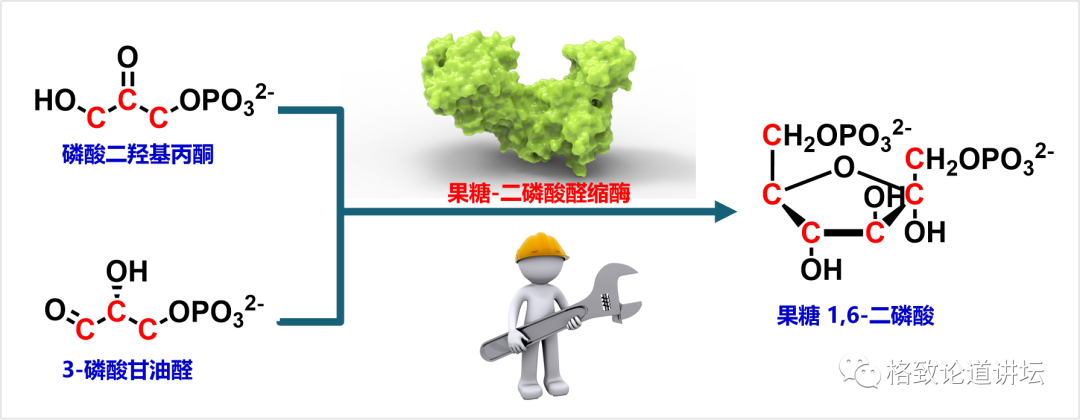
However, once we add an enzyme in this system, such as fructose dipholtosphosylinopase, we can significantly increase the speed of the reaction. This process may be completed within a few minutes. We can compare enzymes as a worker with special skills on the factory assembly line. It can efficiently complete a very complicated and challenging thing.
A 9 -step reaction enzyme was found from different creatures, but ...
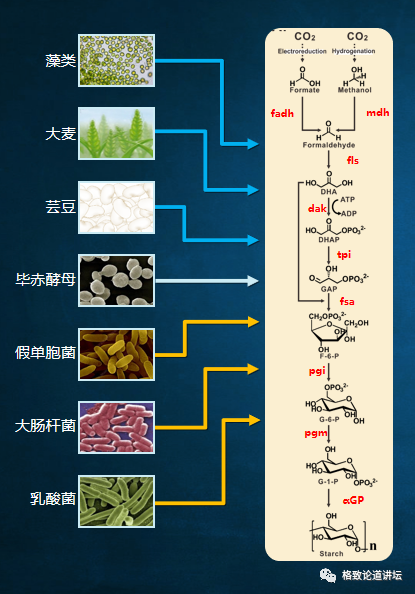
Therefore, we need to find enzymes that catalyzes these reactions. We add these enzymes to add chemical reactions and expect to see starch. But things do not have a lot of optimization and adjustment, but we have never seen the starch in hope. What is the reason?
After our analysis, we believed that because these enzymes came from different species, they never worked together. We rashly put them together for them to work together, and there may be problems in the meantime.
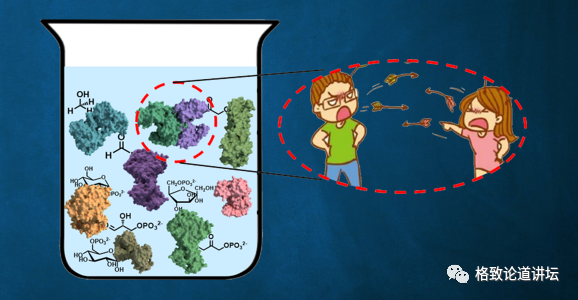
where is the problem?
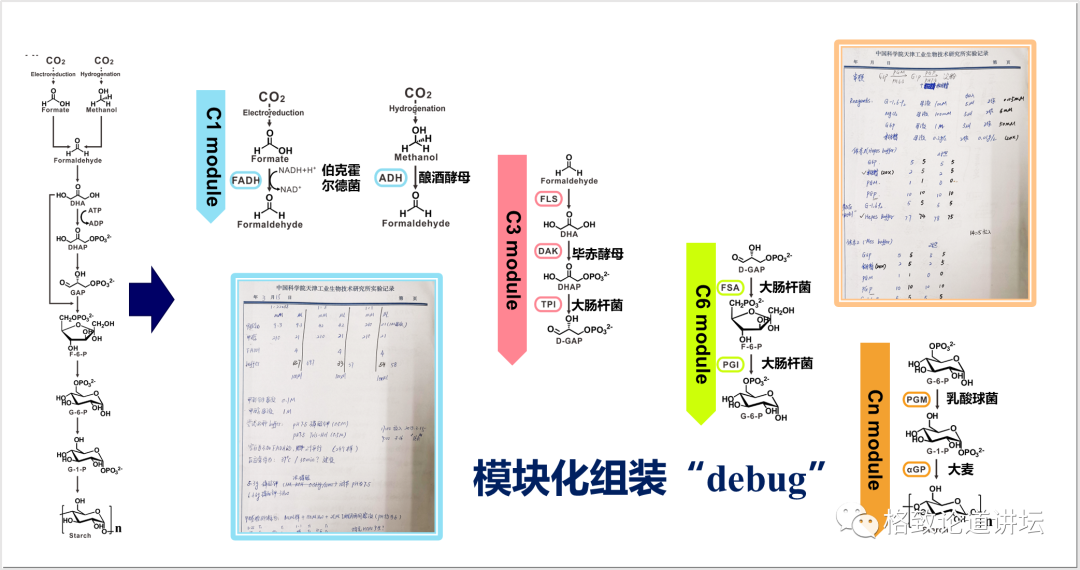
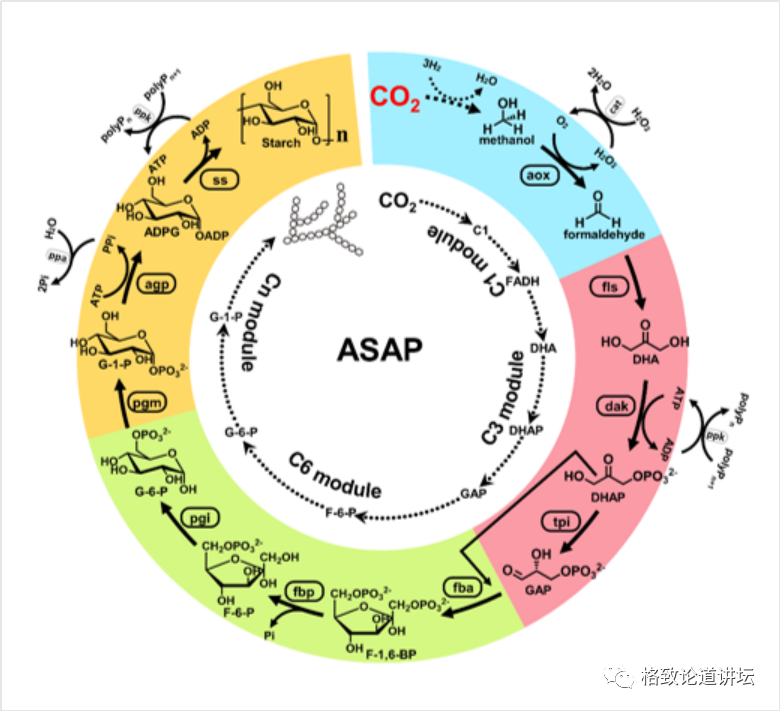
Therefore, we need to find these issues. We borrowed the modular concept in software programming and cut very complicated manual computing into 4 simpler modules. In each module, we designed a very convenient way to detect their functions. The picture in the lower right corner and the upper left corner is an experiment we did when testing the module in 2016. It looks complicated, in fact, it is very simple to say. We have recipes during cooking. For example, one dish requires two tomatoes, three eggs, a little salt, and two spoons of soy sauce. According to the temperature and time on the recipe, you can taste it. Our job is actually similar to cooking. For example, in this experimental record, how much do we write down the enzyme, how much the substrate is placed, the temperature should be controlled at 30 degrees, and the time may be half an hour.
From virtual to reality: 9 steps to 11 steps
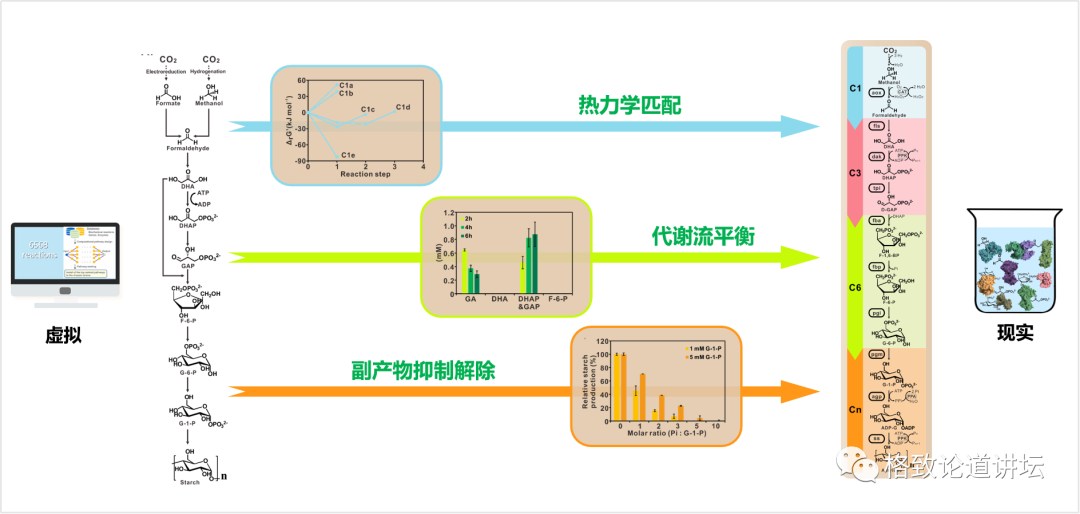
After that, we can detect the final product through chemical and physical methods. We found that the place where the problem is really not in the module is between the modules. For example, the blue and red modules in the figure, that is, the three modules of carbon one and carbon, actually have the problem of incomparable thermodynamics. There is a problem of metabolic flow between the carbon three and carbon six modules. There is a problem of by -product suppression in the carbon six and carbon N modules. After discovering these problems, we adjusted the corresponding modules and re -designed. In this way, we have transformed a total of 9 steps that were originally designed by the computer to be a 9 -step reaction that can be achieved in a cup or test tube. The whole process was compared with 62 enzymes from 31 species. July 24, 2018 is the day I have always remembered, because after more than three years of work, we saw starch blue for the first time.
The starch we usually see is white powder, but the starch has a very typical color reaction, that is, the iodine will turn blue. In this way, we can judge whether starch is generated. The far left is a negative control group, which we call a negative control. It does not produce any starch, and it appears white after iodine. The right is a positive contrast. When many starch is synthesized, this is the dark blue after iodine dye. In the middle is a sample that we have adjusted many times, it is a very light and weak blue.
This is because we have a short starch chain in the initial synthesis. The shorter the chain of starch, the more it biases its color to purple -red, and the longer it is, it is biased to dark blue. And the amount of our first synthesis is very low, so the blue is very weak. Although the color is very weak, it has unparalleled vitality in our eyes. Because we know that it represents a brand new possibility, a possibility that no longer needs plants to synthesize starch.

3
Artificial synthetic starch: from 1.0 to 3.0
So far, we have built a 1.0 version of the synthetic pathway of artificial starch, which realizes the leap from virtual to reality.
Artificial Starch Anabolic Pathway (Asap 1.0)
The entire path is composed of 1 step chemical reactions and 10 -step bio -reactions. However, at this stage, the production intensity of starch is relatively low, about only 3 mg per liter per hour, which is slower than the synthetic rate of plants.
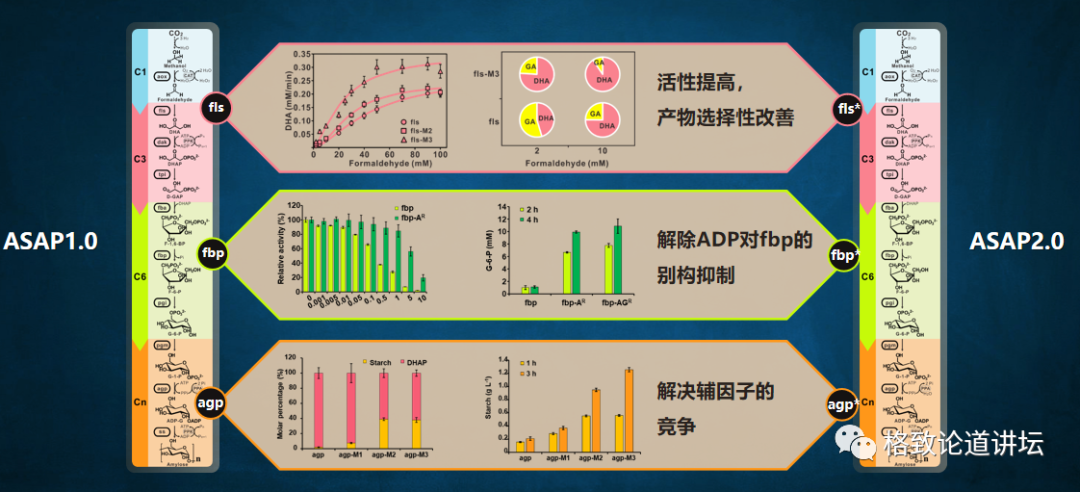
We analyzed the reason and found that there were 3 enzymes in this approach, and their activity would be more or less affected by various or less, which caused the starch synthesis capacity of the entire path to decline significantly. This is like a worker with insufficient business capabilities on the production line. As soon as the product arrives, the shell is stuck, resulting in a significant decline in the efficiency of the entire production line. How to do? We can train workers to improve his business level. Similarly, we can transform enzymes to improve its performance.
This picture is a transformation of enzymes. Enzymes are formed by amino acid sequences, and they have some key amino acids in its catalytic center, which plays a decisive role in catalytic. We turn a very critical 28 -bit alcoholicine into brilliantine, which makes the channels smaller, and then improve the enzyme activation of enzymes.
In a similar way, we transformed the three key enzymes in this way. The first enzyme is FLS. By transforming, we improve its activity; the second enzyme is FBP. We relieved the feedback suppression of the auxiliary factor ADP. The competitiveness of ADP's auxiliary factor. By the transformation of these 3 enzymes, we have built a 2.0 version of artificial starch synthesis pathway. The starch synthesis strength of version 2.0 has been increased by nearly 8 times.

At this step, we have completed the synthesis work from one carbon to starch. Below we need to think about how to integrate the steps of carbon dioxide chemistry, because our final goal is to synthesize starch through carbon dioxide. At this time we found the team of Academician Li Can.
Left: Photovoltaic electrolyte hydrogen control: CO2 hydrogen methanol production methanol
Academician Li's team has been committed to studying the conversion and utilization of carbon dioxide in more than 20 years. The "liquid sunlight" plan he proposed is actually to turn solar energy through photovoltaic power, and then electrolytic water generates hydrogen. Synthetic methanol. Methanol can be used as fuel or raw materials for other compound synthesis. The entire route can make the efficiency of solar energy more chemical energy than 10%, which is far more than natural photosynthesis.
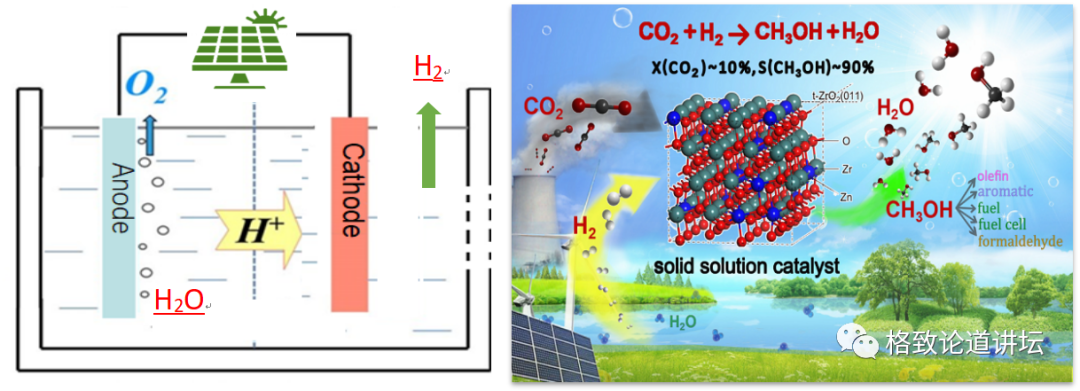
Academician Li provided us with a lot of help. But chemical reactions are a process of high temperature and high pressure, and it is faster. And creatures should react under mild conditions, and it is slower as a whole. This causes many intermediate products from the synthesis of methanol to the biological system, which will have a very serious inhibitory effect on enzymes.
Therefore, we propose a strategy of space -time separation, which is to separate chemical reactions and biological reactions for space. After the chemical reactions generate methanol, we first synthesized the three -carbon DHA, and then the subsequent reaction was performed to synthesize starch from DHA. Through this process, we have built a 3.0 version of artificial starch synthesis pathway.
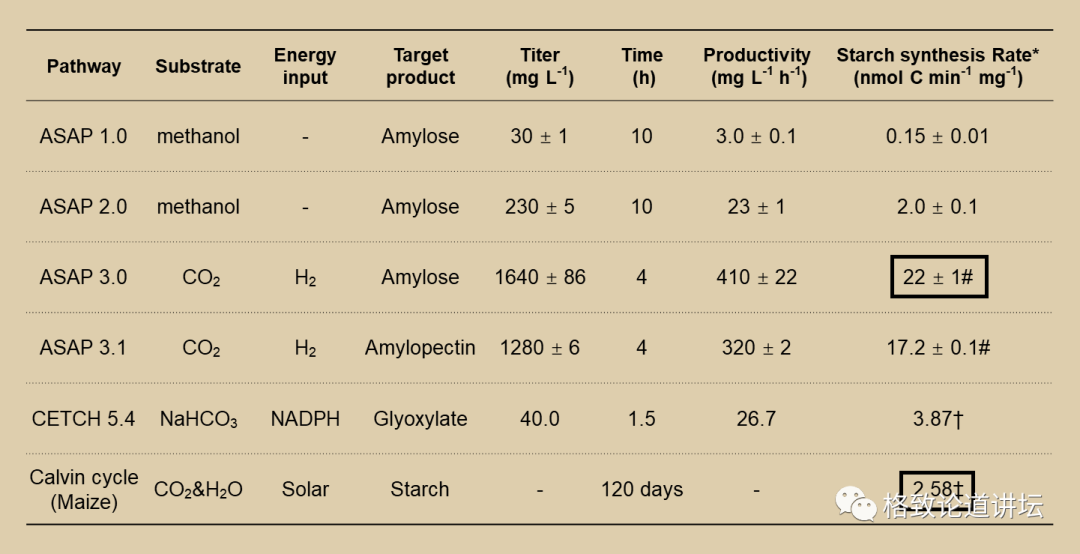
The intensity of the starch synthesis of version 3.0 has nearly 20 times higher than that of version 2.0. What's more, in this model, we can not only synthesize straight starch, but also synthesize branches of starch. In the bottle in the upper right corner, the dark blue is straight starch, and the reddish -brown starch is stained. Among the plants in nature, these two starch are mixed together, and we are equivalent to achieving these two starch controllable synthesis. It took us 3 years to achieve artificial synthetic starch from scratch. We created ASP1.0 version, and it took more than 3 years to increase the starch synthesis capacity by 136 times.
At the bottom is the starch synthesis rate of corn
At present, the carbon conversion rate of version 3.0 is already 8.5 times that of corn starch synthesis in nature. But I want to say that version 3.0 is not our final version, it is just a process. Later we also have 4.0 and 5.0 versions to continuously improve the energy conversion efficiency of manual channels and the synthetic rate of starch.
Looking back at the past six years, we have recorded a total of 33 experiments and recorded more than 2,000 days and nights of we manually synthetic starch. Among them, there is the joy of success and the frustration of failure.
4
What will artificial synthetic starch bring?

Many people ask us, can artificial synthetic starch be eaten? Frankly speaking, I can't answer this question yet.
Because the starch synthesized in the laboratory is only about 1 gram, we don't dare to eat it, we are really reluctant to eat. So one of our latter work is to accelerate its industrialization application.
This process is not simply enlarged, we also have to solve some basic scientific issues of its entire enlarged process. It is hoped that in the future, we can produce kg -level or even tons of starch. At that time, we can answer this question.
Our current work is still in the laboratory stage. If we really want to realize the use of industrialization, we still face many difficulties and challenges, but this does not prevent us from thinking about what impact this technology can have on our lives in the future.
I think the impact of the first time is the change of the agricultural model. We can no longer need a large -scale planting field to achieve starch production. We can also use this method or this type of technology to produce a series of traditional agricultural products including starch, protein, oil, etc.
The other is the impact on the chemical industry. Our traditional chemical industry depends on petrochemical resources, and such technologies can produce starch and various chemicals with carbon dioxide as raw materials, and establish a new industrial route with carbon dioxide as raw materials.
In addition, we often look up at the starry sky and explore the universe is the ultimate dream of human beings. But what do we eat after leaving the earth? Such technologies can be synthesized by starch, protein, and various food components in a small space to provide guarantee for us to survive.

This job is the result of joint research together by multiple teams. I would like to thank Academician Li Can, Academician Zhao Guoping and many other experts and seniors for their guidance and help to us. I also have to thank the Chinese Academy of Sciences and Tianjin for their support for basic research. When we have only one idea, we have given key deployment projects to support us to complete our dreams.
In addition, I also want to thank our research institute's mechanism system innovation so that we can not worry about the publication of the article, the fight for funding and the promotion of the title, so that I can calm down for six years to do this work. In the end, I would like to thank the director Ma Yanhe. It was his long -awaited long -sightedness that gave me the opportunity to realize the starch blueprint.
Although our 6 -year -old task is completed, the story of artificial synthesis starch has not ended. If you are interested in the direction of artificially synthetic starch, please join us, because you have a blue youth dream, you will be more exciting. thank you all!

Uncle Ku Welfare
Uncle Ku's book is always there! Boji Tianjuan provided Uncle Ku with 20 "Pain" to be given enthusiastic readers. Douban 9.4 -point ultra -high word -of -mouth British drama "Pain is inevitable" original novel! A male doctor who is absolutely ecstatic to be ecstatic! It was burnt and funeral, with an average of 1 smile every 3 sentences, and was deeply cured after laughing. Please comment under the article that the top 3 (more than 50) who likes the highest likes will get a book.


- END -
Maker "Hui" gathered together to light up the dream of entrepreneurial!The Eleventh China Innovation and Entrepreneurship Competition Henan Division Jiaozuo Branch Finals Startup

Henan Daily Client Reporter Ji Jian Correspondent Xue JingOn August 3, the reporte...
The nation's first "Weihai Preparation Insurance" platform is upgraded again
